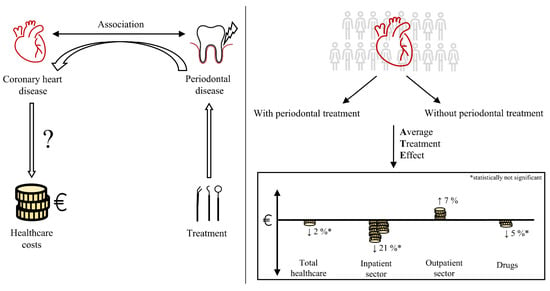
Association between Periodontal Treatment and Healthcare Costs in Patients with Coronary Heart Disease: A Cohort Study Based on German Claims Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposure to Periodontal Treatment
2.3. Outcome
2.4. Confounders
2.5. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef]
- Jordan, A.R.; Micheelis, W. Fünfte Deutsche Mundgesundheitsstudie (DMS V); Deutscher Zahnärzte Verlag DÄV: Köln, Germany, 2016. [Google Scholar]
- Zhu, K.-F.; Wang, Y.-M.; Zhu, J.-Z.; Zhou, Q.-Y.; Wang, N.-F. National prevalence of coronary heart disease and its relationship with human development index: A systematic review. Eur. J. Prev. Cardiol. 2020, 23, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch-Institut (Ed.) Gesundheit in Deutschland: Gesundheitsberichterstattung des Bundes. In Gemeinsam getragen von RKI und Destatis; RKI: Berlin, Germany, 2015. [Google Scholar]
- Dannewitz, B.; Holtfreter, B.; Eickholz, P. Periodontitis—Therapy of a widespread disease. Bundesgesundheitsblatt Gesundh. Gesundh. 2021. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Seitz, M.W.; Listl, S.; Bartols, A.; Schubert, I.; Blaschke, K.; Haux, C.; Van Der Zande, M.M. Current Knowledge on Correlations Between Highly Prevalent Dental Conditions and Chronic Diseases: An Umbrella Review. Prev. Chronic. Dis. 2019, 16, E132. [Google Scholar] [CrossRef] [Green Version]
- Sanz, M.; Marco del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Falcao, A.; Bullon, P. A review of the influence of periodontal treatment in systemic diseases. Periodontol 2000 2019, 79, 117–128. [Google Scholar] [CrossRef]
- Gao, S.; Tian, J.; Li, Y.; Liu, T.; Li, R.; Yang, L.; Xing, Z. Periodontitis and Number of Teeth in the Risk of Coronary Heart Disease: An Updated Meta-Analysis. Med. Sci. Monit. 2021, 27, e930112. [Google Scholar] [CrossRef] [PubMed]
- Meurman, J.H.; Söder, B. The Stockholm Study: Over 30 years’ Observation of the Effect of Oral Infections on Systemic Health. Dent. J. 2022, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Bolger, A.F.; Papapanou, P.N.; Osinbowale, O.; Trevisan, M.; Levison, M.E.; Taubert, K.A.; Newburger, J.W.; Gornik, H.L.; Gewitz, M.H.; et al. Periodontal Disease and Atherosclerotic Vascular Disease: Does the Evidence Support an Independent Association? A Scientific Statement From the American Heart Association. Circulation 2012, 125, 2520–2544. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, N.; Loos, B.G. Periodontitis and atherosclerotic cardiovscular disease. In Periodontitis and Systemic Diseases: Clinical Evidence and Biological Plausibility; Hirschfeld, J., Chapple, I.L.C., Eds.; Quintessenz Verlags-GmbH: Berlin, Germany, 2021. [Google Scholar]
- Joshi, C.; Bapat, R.; Anderson, W.; Dawson, D.; Cherukara, G.; Hijazi, K. Serum antibody response against periodontal bacteria and coronary heart disease: Systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 1570–1586. [Google Scholar] [CrossRef]
- Irwandi, R.A.; Kuswandani, S.O.; Harden, S.; Marletta, D.; D’Aiuto, F. Circulating inflammatory cell profiling and periodontitis: A systematic review and meta-analysis. J. Leukoc. Biol. 2022, 111, 1069–1096. [Google Scholar] [CrossRef]
- Teeuw, W.J.; Slot, D.E.; Susanto, H.; Gerdes, V.E.; Abbas, F.; D’Aiuto, F.; Kastelein, J.J.; Loos, B.G. Treatment of periodontitis improves the atherosclerotic profile: A systematic review and meta-analysis. J. Clin. Periodontol. 2014, 41, 70–79. [Google Scholar] [CrossRef] [PubMed]
- D’Aiuto, F.; Orlandi, M.; Gunsolley, J.C. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J. Clin. Periodontol. 2013, 40, S85–S105. [Google Scholar] [CrossRef]
- Vandenberghe, D.; Albrecht, J. The financial burden of non-communicable diseases in the European Union: A systematic review. Eur. J. Public Health 2020, 30, 833–839. [Google Scholar] [CrossRef]
- Righolt, A.J.; Jevdjevic, M.; Marcenes, W.; Listl, S. Global-, Regional-, and Country-Level Economic Impacts of Dental Diseases in 2015. J. Dent. Res. 2018, 97, 501–507. [Google Scholar] [CrossRef]
- Albert, D.A.; Sadowsky, D.; Papapanou, P.; Conicella, M.L.; Ward, A. An examination of periodontal treatment and per member per month (PMPM) medical costs in an insured population. BMC Health Serv. Res. 2006, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Jeffcoat, M.K.; Jeffcoat, R.L.; Gladowski, P.A.; Bramson, J.B.; Blum, J.J. Impact of Periodontal Therapy on General Health: Evidence from Insurance Data for Five Systemic Conditions. Am. J. Prev. Med. 2014, 47, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Andersohn, F.; Walker, J. Characteristics and external validity of the German Health Risk Institute (HRI) Database. Pharmacoepidemiol. Drug Saf. 2016, 25, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Schubert, I.; Ihle, P.; Köster, I. Internal Confirmation of Diagnoses in Routine Statutory Health Insurance Data: Concept with Examples and Case Definitions. Gesundheitswesen 2010, 72, 316–322. [Google Scholar] [CrossRef]
- Kassenzahnärztliche Bundesvereinigung (KZBV); Spitzenverband Bund der Krankenkassen (GKV-Spitzenverband). Einheitlicher Bewertungsmaßstab für Zahnärztliche Leistungen Gemäß § 87 Abs. 2 und 2h SGB V (BEMA): Anlage A zum Bundesmantelvertrag—Zahnärzte (BMV-Z); KZBV: Cologne, Germany; GKV-Spitzenverband: Berlin, Germany, 2019. [Google Scholar]
- Robert Koch-Institut (Ed.) Daten und Fakten: Ergebnisse der Studie »Gesundheit in Deutschland aktuell 2012«. Beiträge zur Gesundheitsberichterstattung des Bundes; RKI: Berlin, Germany, 2014. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Emsley, R.; Lunt, M.; Pickles, A.; Dunn, G. Implementing double-robust estimators of causal effects. Stata J. 2008, 8, 334–353. [Google Scholar] [CrossRef] [Green Version]
- Nasseh, K.; Vujicic, M.; Glick, M. The Relationship between Periodontal Interventions and Healthcare Costs and Utilization. Evidence from an Integrated Dental, Medical, and Pharmacy Commercial Claims Database. Health Econ. 2017, 26, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, K.; Hellmich, M.; Samel, C.; Listl, S.; Schubert, I. The impact of periodontal treatment on healthcare costs in newly diagnosed diabetes patients: Evidence from a German claims database. Diabetes Res. Clin. Pract. 2020, 172, 108641. [Google Scholar] [CrossRef]
- Garrido, M.M.; Kelley, A.S.; Paris, J.; Roza, K.; Meier, D.E.; Morrison, R.S.; Aldridge, M.D. Methods for constructing and assessing propensity scores. Health Serv. Res. 2014, 49, 1701–1720. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.T.; Yu, T.M.; Ke, T.Y.; Wu, M.J.; Chuang, Y.W.; Li, C.Y.; Chiu, C.W.; Lin, C.L.; Liang, W.M.; Chou, T.C.; et al. Intensive Periodontal Treatment Reduces Risks of Hospitalization for Cardiovascular Disease and All-Cause Mortality in the Hemodialysis Population. J. Clin. Med. 2018, 7, 344. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, S.; Suvan, J.; Deschner, J. The association of periodontal diseases with metabolic syndrome and obesity. Periodontol 2000. 2020, 83, 125–153. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Parodontologie (DG PARO); Deutsche Gesellschaft für Zahn-, Mund- und Kieferheilkunde (DGZMK). S3-Leitlinie (Langversion): Die Behandlung von Parodontitis Stadium I bis III—Die deutsche Implementierung der S3-Leitlinie “Treatment of Stage I–III Periodontitis“ der European Federation of Periodontology (EFP). Available online: https://www.awmf.org/uploads/tx_szleitlinien/083-043l_S3_Behandlung-von-Parodontitis-Stadium-I-III_2021-02_2.pdf (accessed on 8 July 2022).
- Schulz, S.; Schlitt, A.; Hofmann, B.; Schaller, H.G.; Reichert, S. Periodontal pathogens and their role in cardiovascular outcome. J. Clin. Periodontol. 2020, 47, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German Cardiac Society (DGK). Die PARO-CHD-Studie: Eine (schwere) Parodontitis ist bei Patienten mit koronarer Herzkrankheit aus Ostdeutschland Häufig, Steht aber Nicht in Zusammenhang zu Zukünftigen Kardiovaskulären Ereignissen (Pressetext). Available online: https://dgk.org/daten/Schlitt-Paro-CHD.pdf (accessed on 19 May 2021).
- Sekundo, C.; Bölk, T.; Kalmus, O.; Listl, S. Accuracy of a 7-Item Patient-Reported Stand-Alone Tool for Periodontitis Screening. J. Clin. Med. 2021, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, M.J.L.; Teeuw, W.J.; Bizzarro, S.; Muris, J.; Su, N.; Nicu, E.A.; Nazmi, K.; Bikker, F.J.; Loos, B.G. A rapid, non-invasive tool for periodontitis screening in a medical care setting. BMC Oral Health 2019, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Smits, K.; Kalmus, O.; Haux, C.; Seitz, M.W.; Van der Zande, M.M.; Schubert, I.; Listl, S. Towards a decision support system to better inegrate primary and dental care. Int. J Integr. Care 2019, 19, 479. [Google Scholar] [CrossRef] [Green Version]
- Hierse, L. Parodontitis als Volkskrankheit. Prävalenz, Diagnostik, Therapie und eine kritische Auseinandersetzung mit der Kostenübernahme durch die GKV. IGZ DIE ALTERNATIVE 2015, 1, 4–10. [Google Scholar]
- Chowdhury, T.T.; Hemmelgarn, B.R. Evidence-Based Decision Making 6: Administrative Databases as Secondary Data Source for Epidemiologic and Health Service Research. In Clinical Epidemiology: Practice and Methods; Parfrey, P.S., Barrett, B.J., Eds.; Springer Science+Business Media: New York, NY, UAS, 2021; Volume 3. [Google Scholar]
- Park, S.Y.; Kim, S.H.; Kang, S.H.; Yoon, C.H.; Lee, H.J.; Yun, P.Y.; Youn, T.J.; Chae, I.H. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: A population-based study from Korea. Eur. Heart J. 2019, 40, 1138–1145. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Maiorani, C.; Preda, C.; Chiesa, A.; Esposito, F.; Pascadopoli, M.; Scribante, A. Management of Gingival Bleeding in Periodontal Patients with Domiciliary Use of Toothpastes Containing Hyaluronic Acid, Lactoferrin, or Paraprobiotics: A Randomized Controlled Clinical Trial. Appl. Sci. 2021, 11, 8586. [Google Scholar] [CrossRef]
- Ghasemi, N.; Behnezhad, M.; Asgharzadeh, M.; Zeinalzadeh, E.; Kafil, H.S. Antibacterial Properties of Aloe vera on Intracanal Medicaments against Enterococcus faecalis Biofilm at Different Stages of Development. Int. J. Dent. 2020, 2020, 8855277. [Google Scholar] [CrossRef]
- Gemeinsamer Bundesausschuss (G-BA). Richtlinie des Gemeinsamen Bundesausschusses zur Systematischen Behandlung von Parodontitis und Anderer Parodontalerkrankungen (PAR-Richtlinie) in der Fassung vom 17. Dezember 2020, Veröffentlicht im Bundesanzeiger am 21. Juni 2021 (BAnz AT 21.06.2021 B2), in Kraft Getreten am 1 Juli 2021, Geändert am 16 Dezember 2021, Veröffentlicht im Bundesanzeiger (BAnz AT 12.05.2022 B2), in Kraft Getreten am 13. Mai 2022. Available online: https://www.g-ba.de/downloads/62-492-2817/PAR-RL_2021-12-16_iK-2022-05-13.pdf (accessed on 8 July 2022).


| Periodontal Treatment | ||||
|---|---|---|---|---|
| Total | Exposed Group | Unexposed Group | ||
| N (%) | 21,263 (100) | 1003 (4.7) | 20,260 (95.3) | |
| Sex, n (%) | Men | 12,903 (60.7) | 646 (64.4) | 12,257 (60.5) |
| Women | 8360 (39.3) | 357 (35.6) | 8003 (39.5) | |
| Age, mean (SD) | 64 (12.5) | 60 (10.5) | 64 (12.5) | |
| Nielsen region, n (%) | Bavaria | 2926 (13.8) | 132 (13.2) | 2794 (13.8) |
| Baden-Wuerttemberg | 2532 (11.9) | 129 (12.9) | 2403 (11.9) | |
| Centre | 3472 (16.3) | 131 (13.1) | 3341 (16.5) | |
| North (West) | 2772 (13.0) | 125 (12.5) | 2647 (13.1) | |
| North Rhine-Westphalia | 7735 (36.4) | 416 (41.5) | 7319 (36.1) | |
| East (North) | 1252 (5.9) | 53 (5.3) | 1199 (5.9) | |
| East (South) | 545 (2.6) | 16 (1.6) | 529 (2.6) | |
| Missing | 29 (0.1) | <5 * | <29 (0.1) | |
| Dental visit pre, n (%) | Yes | 14,963 (70.4) | 825 (82.3) | 14,138 (69.8) |
| No | 6300 (29.6) | 178 (17.7) | 6122 (30.2) | |
| Charlson comorbidity index, mean (SD) | 2.0 (1.9) | 1.6 (1.7) | 2.0 (1.9) | |
| Physician group visits pre, mean (SD) | 5.0 (3.0) | 5.0 (3.2) | 5.0 (3.0) | |
| Total healthcare costs pre, mean (SD) | 2517.8 (4882.3) | 1998.5 (3660.1) | 2543.5 (4933.6) | |
| Costs | Periodontal Treatment | Min. | Q25 | Median | Q75 | Max. | Mean | Geom. Mean |
|---|---|---|---|---|---|---|---|---|
| Total healthcare costs, € | Exposed group | 0 | 592 | 1136 | 2878 | 85,976 | 2950 | 1293 |
| Unexposed group | 0 | 663 | 1295 | 3413 | 233,167 | 3488 | 1483 | |
| Inpatient costs, € | Exposed group | 0 | 0 | 0 | 619 | 84,778 | 1643 | 12 |
| Unexposed group | 0 | 0 | 0 | 1613 | 231,593 | 2051 | 17 | |
| Outpatient costs, € | Exposed group | 0 | 412 | 685 | 1107 | 14,811 | 920 | 627 |
| Unexposed group | 0 | 437 | 709 | 1136 | 25,543 | 921 | 656 | |
| Drug costs, € | Exposed group | 0 | 72 | 160 | 403 | 19,053 | 386 | 169 |
| Unexposed group | 0 | 86 | 210 | 527 | 80,618 | 516 | 174 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaschke, K.; Hellmich, M.; Samel, C.; Listl, S.; Schubert, I. Association between Periodontal Treatment and Healthcare Costs in Patients with Coronary Heart Disease: A Cohort Study Based on German Claims Data. Dent. J. 2022, 10, 133. https://doi.org/10.3390/dj10070133
Blaschke K, Hellmich M, Samel C, Listl S, Schubert I. Association between Periodontal Treatment and Healthcare Costs in Patients with Coronary Heart Disease: A Cohort Study Based on German Claims Data. Dentistry Journal. 2022; 10(7):133. https://doi.org/10.3390/dj10070133
Chicago/Turabian StyleBlaschke, Katja, Martin Hellmich, Christina Samel, Stefan Listl, and Ingrid Schubert. 2022. "Association between Periodontal Treatment and Healthcare Costs in Patients with Coronary Heart Disease: A Cohort Study Based on German Claims Data" Dentistry Journal 10, no. 7: 133. https://doi.org/10.3390/dj10070133