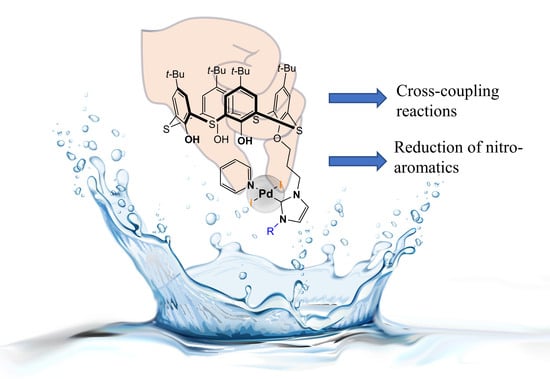
PEPPSI-Type Pd(II)—NHC Complexes on the Base of p-tert-Butylthiacalix[4]arene: Synthesis and Catalytic Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Catalytic Activities
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, Q.; Li, J.; Wen, X.; Huang, Y.; Hu, Y.; Huang, Q.; Xu, G.; Xie, Y.; Zhou, H. Carbohydrate-substituted N-heterocyclic carbenes Palladium complexes: High efficiency catalysts for aqueous Suzuki–Miyaura reaction. Carbohydr. Res. 2022, 512, 108516. [Google Scholar] [CrossRef]
- Farhang, M.; Akbarzadeh, A.R.; Rabbani, M.; Ghadiri, A.M. A retrospective-prospective review of Suzuki–Miyaura reaction: From cross-coupling reaction to pharmaceutical industry applications. Polyhedron 2022, 227, 116124. [Google Scholar] [CrossRef]
- Al Nasr, I.; Touj, N.; Koko, W.; Khan, T.; Özdemir, I.; Yaşar, S.; Hamdi, N. Biological activities of nhc–pd(Ii) complexes based on benzimidazolylidene n-heterocyclic carbene (nhc) ligands bearing aryl substituents. Catalysts 2020, 10, 1190. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Chang, S.Y.; Ejaz, M.; Samy, M.M.; Mousa, A.O.; Kuo, S.W. Design and Synthesis of Bisulfone-Linked Two-Dimensional Conjugated Microporous Polymers for CO2 Adsorption and Energy Storage. Molecules 2023, 28, 3234. [Google Scholar] [CrossRef] [PubMed]
- Koy, M.; Bellotti, P.; Das, M.; Glorius, F. N-Heterocyclic carbenes as tunable ligands for catalytic metal surfaces. Nat. Catal. 2021, 4, 352–363. [Google Scholar] [CrossRef]
- Valente, C.; Çalimsiz, S.; Hoi, K.H.; Mallik, D.; Sayah, M.; Organ, M.G. The development of bulky palladium NHC complexes for the most-challenging cross-coupling reactions. Angew. Chem. Int. Ed. 2012, 51, 3314–3332. [Google Scholar] [CrossRef]
- Smith, C.A.; Narouz, M.R.; Lummis, P.A.; Singh, I.; Nazemi, A.; Li, C.H.; Crudden, C.M. N-Heterocyclic Carbenes in Materials Chemistry. Chem. Rev. 2019, 119, 4986–5056. [Google Scholar] [CrossRef]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 nobel prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Vasu, G.R.P.; Venkata, K.R.M.; Kakarla, R.R.; Ranganath, K.V.S.; Aminabhavi, T.M. Recent advances in sustainable N-heterocyclic carbene-Pd(II)-pyridine (PEPPSI) catalysts: A review. Environ. Res. 2023, 225, 115515. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, G.; Guob, D.; Wang, J. Recent developments on NHC-driven dual catalytic approaches. Org. Chem. Front. 2022, 9, 5016–5040. [Google Scholar] [CrossRef]
- Boztepe, C.; Künkül, A.; Gürbüz, N. Hydrogel supported vinylimidazole based PEPPSI-Pd-NHC catalysts: The catalytic activities in Heck and Suzuki-Miyaura coupling reactions. J. Mol. Struct. 2020, 1209, 127948. [Google Scholar] [CrossRef]
- Kaloğlu, N.; Özdemir, İ. PEPPSI-Pd-NHC catalyzed Suzuki-Miyaura cross-coupling reactions in aqueous media. Tetrahedron 2019, 75, 2306–2313. [Google Scholar] [CrossRef]
- Eremin, D.B.; Denisova, E.A.; Kostyukovich, A.Y.; Martens, J.; Berden, G.; Oomens, J.; Khrustalev, V.N.; Chernyshev, V.M.; Ananikov, V.P. Ionic Pd/NHC Catalytic System Enables Recoverable Homogeneous Catalysis: Mechanistic Study and Application in the Mizoroki–Heck Reaction. Chem. Eur. J. 2019, 25, 16564–16572. [Google Scholar] [CrossRef]
- Korukçu, M.Ç.; Can, S. Pd–PEPPSI type complexes bearing unsymmetrical NHC ligand with phenyl-substituted backbone: Highly efficient catalysts for Heck–Mizoroki and Suzuki–Miyaura cross-coupling reactions Meliha. Appl. Organomet. Chem. 2023, 37, 7057. [Google Scholar] [CrossRef]
- Lima, C.G.S.; Pauli, F.P.; Madriaga, V.G.; Amaral, A.A.P.; Graciano, I.A.; Meira, V.L.; Forezi, L.d.S.M.; Ferreira, V.F.; Lima, T.d.M.; de Carvalho da Silva, F. Supramolecular Catalysts for Organic Synthesis: Preparation and Applications of Cyclodextrins and Calixarenes in C−C Cross-Coupling Reactions. Eur. J. Org. Chem. 2022, 2022, e202200904. [Google Scholar] [CrossRef]
- Snelders, D.J.M.; Van Koten, G.; Klein Gebbink, R.J.M. Hexacationic Dendriphos ligands in the Pd-catalyzed Suzuki-Miyaura cross-coupling reaction: Scope and mechanistic studies. J. Am. Chem. Soc. 2009, 131, 11407–11416. [Google Scholar] [CrossRef]
- Jover, J.; Fey, N.; Purdie, M.; Lloyd-Jones, G.C.; Harvey, J.N. A computational study of phosphine ligand effects in Suzuki-Miyaura coupling. J. Mol. Catal. A Chem. 2010, 324, 39–47. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Wang, Y.; Wang, J. Synthesis, structure and catalysis/applications of N-heterocyclic carbene based on macrocycles. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 15–37. [Google Scholar] [CrossRef]
- Almallah, H.; Nos, M.; Ayzac, V.; Brenner, E.; Matt, D.; Gourlaouen, C.; Jahjah, M.; Hijazi, A. Complexes featuring N-heterocyclic carbenes with bowl-shaped wingtips. Comptes Rendus Chim. 2019, 22, 299–309. [Google Scholar] [CrossRef]
- Şahin, N.; Semeril, D.; Brenner, E.; Matt, D.; Kaya, C.; Toupet, L. Palladium-catalysed Suzuki-Miyaura cross-coupling with imidazolylidene ligands substituted by crowded resorcinarenyl and calixarenyl units. Turk. J. Chem. 2015, 39, 1171–1179. [Google Scholar] [CrossRef]
- Kaloğlu, M.; Sémeril, D.; Brenner, E.; Matt, D.; Özdemir, I.; Toupet, L. The Influence of Imidazolylidene Ligands with Bulky Resorcinarenyl Substituents on Catalysts for Suzuki-Miyaura Coupling. Eur. J. Inorg. Chem. 2016, 2016, 1115–1120. [Google Scholar] [CrossRef]
- Brenner, E.; Matt, D.; Henrion, M.; Teci, M.; Toupet, L. Calix[4]arenes with one and two N-linked imidazolium units as precursors of N-heterocyclic carbene complexes. Coordination chemistry and use in Suzuki-Miyaura cross-coupling. Dalton Trans. 2011, 40, 9889–9898. [Google Scholar] [CrossRef] [PubMed]
- Burilov, V.A.; Gafiatullin, M.B.K.; Mironova, D.A.; Sultanova, E.D.; Evtugyn, V.G.; Osin, Y.N.; Islamov, D.R.; Usachev, K.S.; Solovieva, S.E.; Antipin, I.S. Amphiphilic PdII-NHC Complexes on 1,3-Alternate p-tert-Butylthiacalix[4]arene Platform: Synthesis and Catalytic Activities in Coupling and Hydrogenation Reactions. Eur. J. Org. Chem. 2020, 2020, 2180–2189. [Google Scholar] [CrossRef]
- Gafiatullin, B.K.; Paskevich, I.V.; Burilov, V.A.; Solovieva, S.E.; Antipin, I.S. One-pot Synthesis of Mono-substituted Quaternized p-tert-Butylthiacalix[4]arenes. Macroheterocycles 2022, 15, 53–58. [Google Scholar] [CrossRef]
- Lamouchi, M.; Jeanneau, E.; Coulm, J.; Brioude, A.; Desroches, C. Synthesis and properties of mono-O-[(N-(aminoalkyl)aminocarbonyl)-methoxy] thiacalix[4]arenes and novel mono-O-bridged bisthiacalix[4]arene. Comptes Rendus Chim. 2013, 16, 1073–1078. [Google Scholar] [CrossRef]
- Mague, J.T.; Akkurt, M.; Mohamed, S.K.; Omran, O.A.; Albayati, M.R. N, N, N-Triethylethanaminium 5,11,17,23-tetra- tert -butyl-25-[(ethoxycarbonyl)methoxy]-26,28-dihydroxy-27-oxido-2,8,14,20-tetrathiacalix[4]arene: A molecular salt. IUCrData 2016, 1, x161465. [Google Scholar] [CrossRef]
- Zhao, J.L.; Tomiyasu, H.; Ni, X.L.; Zeng, X.; Elsegood, M.R.J.; Redshaw, C.; Rahman, S.; Georghiou, P.E.; Yamato, T. Synthesis and evaluation of a novel ionophore based on a thiacalix[4]arene derivative bearing imidazole units. New J. Chem. 2014, 38, 6041–6049. [Google Scholar] [CrossRef]
- O’Brien, C.J.; Kantchev, E.A.B.; Valente, C.; Hadei, N.; Chass, G.A.; Lough, A.; Hopkinson, A.C.; Organ, M.G. Easily Prepared Air-and Moisture-Stable Pd–NHC (NHC=N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalyst for the Suzuki–Miyaura Reaction. Chem. Eur. J. 2006, 12, 4743–4748. [Google Scholar] [CrossRef]
- Serdaroğlu, G.; Şahin, N.; Üstün, E.; Tahir, M.N.; Arıcı, C.; Gürbüz, N.; Özdemir, İ. PEPPSI type complexes: Synthesis, x-ray structures, spectral studies, molecular docking and theoretical investigations. Polyhedron 2021, 204, 115281. [Google Scholar] [CrossRef]
- Chernenko, A.Y.; Astakhov, A.V.; Kutyrev, V.V.; Gordeev, E.G.; Burykina, J.V.; Minyaev, M.E.; Khrustalev, V.N.; Chernyshev, V.M.; Ananikov, V.P. Stabilization of the Pd–NHC framework with 1,2,4-triazol-5-ylidene ligands toward decomposition in alkaline media. Inorg. Chem. Front. 2021, 8, 3382–3401. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Joshi, C.; Macharia, J.M.; Izzo, J.A.; Wambua, V.; Kim, S.; Hirschi, J.S.; Vetticatt, M.J. Isotope Effects Reveal the Catalytic Mechanism of the Archetypical Suzuki-Miyaura Reaction. ACS Catal. 2022, 12, 2959–2966. [Google Scholar] [CrossRef]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Selection of boron reagents for Suzuki-Miyaura coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, J.; Singh, S.; Oswal, P.; Arora, A.; Nautiyal, D.; Kumar, A. Suzuki−Miyaura coupling and O−arylation reactions catalysed by palladium(II) complexes of bulky ligands bearing naphthalene core, Schiff base functionality and biarylphosphine moiety. J. Mol. Struct. 2022, 1253, 132099. [Google Scholar] [CrossRef]
- Valente, C.; Belowich, M.E.; Hadei, N.; Organ, M.G. Pd-PEPPSI complexes and the Negishi reaction. Eur. J. Org. Chem. 2010, 2010, 4343–4354. [Google Scholar] [CrossRef]
- Lima, C.F.R.A.C.; Rodrigues, A.S.M.C.; Silva, V.L.M.; Silva, A.M.S.; Santos, L.M.N.B.F. Role of the base and control of selectivity in the suzuki-miyaura cross-coupling reaction. ChemCatChem 2014, 6, 1291–1302. [Google Scholar] [CrossRef]
- Hervés, P.; Pérez-Lorenzo, M.; Liz-Marzán, L.M.; Dzubiella, J.; Lub, Y.; Ballauff, M. Catalysis by metallic nanoparticles in aqueous solution: Model reactions. Chem. Soc. Rev. 2012, 41, 5577–5587. [Google Scholar] [CrossRef]
- Burilov, V.A.; Nugmanov, R.I.; Ibragimova, R.R.; Solovieva, S.E.; Antipin, I.S. “Click chemistry” in the synthesis of new amphiphilic 1,3-alternate thiacalixarenes. Mendeleev Commun. 2015, 25, 177–179. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Zhao, J.; Zhao, Y.; Li, L.; Zhang, H. A Modified Procedure for the Synthesis of 1-Arylimidazoles. Synthesis 2003, 2003, 2661–2666. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van De Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]






| Entry | Complex | Hal | R | Conversion, % | Isolated yield, % | Selectivity, % |
|---|---|---|---|---|---|---|
| 1 | 6 | -I | p-OCH3 | 66 | 53 | 85 |
| 2 | 6 * | 99 | 71 | |||
| 3 | 6 ** | 99 | 65 | |||
| 4 | 6 *** | 99 | 69 | |||
| 5 | 5 | 75 | 61 | 91 | ||
| 6 | 5 * | 99 | 82 | |||
| 7 | 5 ** | 99 | 85 | |||
| 8 | 5 *** | 99 | 84 | |||
| 9 | 4 | 27 | 14 | 96 | ||
| 10 | 4 * | 99 | 91 | |||
| 11 | 4 ** | 99 | 88 | |||
| 12 | 4 *** | 99 | 78 | |||
| 13 | 6 | -I | p-NO2 | 99 | 65 | 99 |
| 14 | 6 * | 99 | 72 | |||
| 15 | 5 | 93 | 35 | 99 | ||
| 16 | 5 * | 99 | 95 | |||
| 17 | 4 | 75 | 35 | 99 | ||
| 18 | 4 * | 95 | 80 | |||
| 19 | 6 | -Br | p-OCH3 | 70 | 48 | 71 |
| 20 | 6 * | 94 | 56 | 71 | ||
| 21 | 5 | 54 | 37 | 75 | ||
| 22 | 5 * | 62 | 42 | 76 | ||
| 23 | 4 | 6 | 3 | 99 | ||
| 24 | 4 * | 18 | 12 | 99 | ||
| 25 | 5 * | -Br | p-CH3 | 56 | 71 | |
| 26 | 5 * | -Mesityl | 23 | 30 | ||
| 27 | 5 * | p-COCH3 | 99 | 98 |
| System | p-Nitrophenol | p-Ethylnitrobenzene | ||
|---|---|---|---|---|
| Apparent Rate Constant, k, s−1 | Specific Catalytic Activity, Ka, × 105 mol1s−1 | Apparent Rate Constant, k, s−1 | Specific Catalytic Activity, Ka, × 105 mol1s−1 | |
| 6 | 2.1 × 10−3 | 4.2 | 2.2 × 10−3 | 4.4 |
| 5 | 4.2 × 10−3 | 8.4 | 2.4 × 10−3 | 4.8 |
| 4 | 1.2 × 10−3 | 2.4 | 2.7 × 10−3 | 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gafiatullin, B.; Akchurina, A.; Fedoseeva, A.; Sultanova, E.; Islamov, D.; Usachev, K.; Burilov, V.; Solovieva, S.; Antipin, I. PEPPSI-Type Pd(II)—NHC Complexes on the Base of p-tert-Butylthiacalix[4]arene: Synthesis and Catalytic Activities. Inorganics 2023, 11, 326. https://doi.org/10.3390/inorganics11080326
Gafiatullin B, Akchurina A, Fedoseeva A, Sultanova E, Islamov D, Usachev K, Burilov V, Solovieva S, Antipin I. PEPPSI-Type Pd(II)—NHC Complexes on the Base of p-tert-Butylthiacalix[4]arene: Synthesis and Catalytic Activities. Inorganics. 2023; 11(8):326. https://doi.org/10.3390/inorganics11080326
Chicago/Turabian StyleGafiatullin, Bulat, Aigul Akchurina, Angelina Fedoseeva, Elza Sultanova, Daut Islamov, Konstantin Usachev, Vladimir Burilov, Svetlana Solovieva, and Igor Antipin. 2023. "PEPPSI-Type Pd(II)—NHC Complexes on the Base of p-tert-Butylthiacalix[4]arene: Synthesis and Catalytic Activities" Inorganics 11, no. 8: 326. https://doi.org/10.3390/inorganics11080326