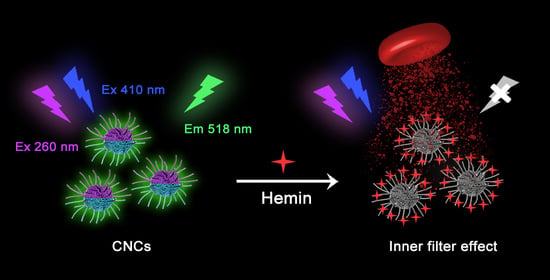
Dual-Exciting Central Carbon Nanoclusters for the Dual-Channel Detection of Hemin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of CNCs
2.2. Optical Properties of CNCs
2.3. Dual-Channel Fluorescent Detection of Hemin
2.4. Determination of Hemin in Cells
3. Materials and Methods
3.1. Materials and Reagents
3.2. Apparatus and Characterization
3.3. Preparation and Purification of CNCs
3.4. Sensing Procedure of Hemin Using CNCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots prepared for fluorescence and chemiluminescence sensing. Sci. China Chem. 2019, 62, 968–981. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, M.L.; Huang, C.Z. Recent advances of carbon dots in imaging-guided theranostics. TrAC Trends Anal. Chem. 2021, 134, 116116. [Google Scholar] [CrossRef]
- Park, S.-H.; Kwon, N.; Lee, J.-H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Bu, X.; Fu, Y.; Wang, Z.; Liu, Q. Recent advances in dual-emission ratiometric fluorescence probes for chemo/biosensing and bioimaging of biomarkers. Coordin. Chem. Rev. 2019, 383, 82–103. [Google Scholar] [CrossRef]
- Chen, B.-B.; Liu, M.-L.; Gao, Y.-T.; Chang, S.; Qian, R.-C.; Li, D.-W. Design and applications of carbon dots-based ratiometric fluorescent probes: A review. Nano Res. 2023, 16, 1064–1083. [Google Scholar] [CrossRef]
- Qian, J.; Ren, C.; Wang, C.; An, K.; Cui, H.; Hao, N.; Wang, K. Gold nanoparticles mediated designing of versatile aptasensor for colorimetric/electrochemical dual-channel detection of aflatoxin B1. Biosens. Bioelectron. 2020, 166, 112443. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Hu, X.; Shi, Y.; Liang, N.; Huang, X.; Wang, X.; Shen, T.; Zou, X.; Shi, J. Simple design concept for dual-channel detection of Ochratoxin A based on bifunctional metal–organic framework. ACS Appl. Mater. Interfaces 2022, 14, 5615–5623. [Google Scholar] [CrossRef]
- Wang, C.; Gao, X.; Wang, S.; Liu, Y. A smartphone-integrated paper sensing system for fluorescent and colorimetric dual-channel detection of foodborne pathogenic bacteria. Anal. Bioanal. Chem. 2020, 412, 611–620. [Google Scholar] [CrossRef]
- Hang, L.; Zhou, F.; Men, D.; Li, H.; Li, X.; Zhang, H.; Liu, G.; Cai, W.; Li, C.; Li, Y. Functionalized periodic Au@MOFs nanoparticle arrays as biosensors for dual-channel detection through the complementary effect of SPR and diffraction peaks. Nano Res. 2017, 10, 2257–2270. [Google Scholar] [CrossRef]
- Fereja, S.L.; Fang, Z.; Li, P.; Guo, J.; Fereja, T.H.; Chen, W. “Turn-off” sensing probe based on fluorescent gold nanoclusters for the sensitive detection of hemin. Anal. Bioanal. Chem. 2021, 413, 1639–1649. [Google Scholar] [CrossRef]
- Gao, L.; Xiao, Y.; Wang, Y.; Chen, X.; Zhou, B.; Yang, X. A carboxylated graphene and aptamer nanocomposite-based aptasensor for sensitive and specific detection of hemin. Talanta 2015, 132, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, R.; Bi, Y.; Qu, H.; Chen, Y.; Zheng, L. Identification of frozen/thawed beef based on label-free detection of hemin (Iron Porphyrin) with solution-gated graphene transistor sensors. Sens. Actuators B Chem. 2020, 305, 127167. [Google Scholar] [CrossRef]
- Du, N.; Zhang, H.; Wang, J.; Dong, X.; Li, J.; Wang, K.; Guan, R. Fluorescent silicon nanoparticle–based quantitative hemin assay. Anal. Bioanal. Chem. 2022, 414, 8223–8232. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, F.; Huang, W.; Bao, H.; Hu, Y.; Huang, X.-a.; Deng, T.; Liu, F. A fluorescence turn-on assay for simple and sensitive determination of hemin and blood stains. Sens. Actuators B Chem. 2020, 304, 127392. [Google Scholar] [CrossRef]
- Ni, P.; Chen, C.; Jiang, Y.; Lu, Y.; Chen, W. A simple and sensitive fluorescent assay for hemin detection based on artemisinin-thiamine. Sens. Actuators B Chem. 2018, 273, 198–203. [Google Scholar] [CrossRef]
- Fereja, T.H.; Kitte, S.A.; Gao, W.; Yuan, F.; Snizhko, D.; Qi, L.; Nsabimana, A.; Liu, Z.; Xu, G. Artesunate-luminol chemiluminescence system for the detection of hemin. Talanta 2019, 204, 379–385. [Google Scholar] [CrossRef]
- Bushira, F.A.; Kitte, S.A.; Xu, C.; Li, H.; Zheng, L.; Wang, P.; Jin, Y. Two-dimensional-plasmon-boosted iron single-atom electrochemiluminescence for the ultrasensitive detection of dopamine, hemin, and mercury. Anal. Chem. 2021, 93, 9949–9957. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Liang, A.; Jiang, Z. SERS and fluorescence dual-mode sensing trace hemin and K+ based on G-quarplex/hemin DNAzyme catalytic amplification. Sens. Actuators B Chem. 2019, 297, 126799. [Google Scholar] [CrossRef]
- Liu, M.L.; Yang, L.; Li, R.S.; Chen, B.B.; Liu, H.; Huang, C.Z. Large-scale simultaneous synthesis of highly photoluminescent green amorphous carbon nanodots and yellow crystalline graphene quantum dots at room temperature. Green Chem. 2017, 19, 3611–3617. [Google Scholar] [CrossRef]
- Chang, S.; Chen, B.B.; Lv, J.; Fodjo, E.K.; Qian, R.C.; Li, D.W. Label-free chlorine and nitrogen-doped fluorescent carbon dots for target imaging of lysosomes in living cells. Microchim. Acta 2020, 187, 435–442. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, Z.X.; Deng, W.C.; Zhan, L.; Liu, M.L.; Huang, C.Z. A large-scale synthesis of photoluminescent carbon quantum dots: A self-exothermic reaction driving the formation of the nanocrystalline core at room temperature. Green Chem. 2016, 18, 5127–5132. [Google Scholar] [CrossRef]
- Gao, Y.-T.; Chen, B.-B.; Jiang, L.; Lv, J.; Chang, S.; Wang, Y.; Qian, R.-C.; Li, D.-W.; Hafez, M.E. Dual-emitting carbonized polymer dots synthesized at room temperature for ratiometric fluorescence sensing of vitamin B12. ACS Appl. Mater. Interfaces 2021, 13, 50228–50235. [Google Scholar] [CrossRef]
- Marković, Z.M.; Labudová, M.; Danko, M.; Matijašević, D.; Mičušík, M.; Nádaždy, V.; Kováčová, M.; Kleinová, A.; Špitalský, Z.; Pavlović, V.; et al. Highly efficient antioxidant F- and Cl-doped carbon quantum dots for bioimaging. ACS Sustain. Chem. Eng. 2020, 8, 16327–16338. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; He, J.H.; Li, C.M.; Li, Y.F.; Huang, C.Z. Anthrax biomarker: An ultrasensitive fluorescent ratiometry of dipicolinic acid by using terbium(III)-modified carbon dots. Talanta 2019, 191, 443–448. [Google Scholar] [CrossRef]
- Stevens, J.S.; de Luca, A.C.; Pelendritis, M.; Terenghi, G.; Downes, S.; Schroeder, S.L.M. Quantitative analysis of complex amino acids and RGD peptides by X-ray photoelectron spectroscopy (XPS). Surf. Interface Anal. 2013, 45, 1238–1246. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Duong, L.V.; Wood, B.J.; Frost, R.L. XPS study of the major minerals in bauxite: Gibbsite, bayerite and (pseudo-) boehmite. J. Colloid Interface Sci. 2006, 296, 572–576. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Gu, J.; Hu, M.J.; Guo, Q.Q.; Ding, Z.F.; Sun, X.L.; Yang, J. High-yield synthesis of graphene quantum dots with strong green photoluminescence. RSC Adv. 2014, 4, 50141–50144. [Google Scholar] [CrossRef]
- Bai, L.; Yan, H.; Feng, Y.; Feng, W.; Yuan, L. Multi-excitation and single color emission carbon dots doped with silicon and nitrogen: Synthesis, emission mechanism, Fe3+ probe and cell imaging. Chem. Eng. J. 2019, 373, 963–972. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Liao, X.; Chen, C.; Yang, J.; Zhou, R.; Si, L.; Huang, Q.; Huang, Z.; Lv, C. Nitrogen-doped carbon dots for dual-wavelength excitation fluorimetric assay for ratiometric determination of phosalone. Microchim. Acta 2021, 188, 247. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Liu, H.; Huang, C.Z.; Ling, J.; Wang, J. Rapid and convenient synthesis of stable silver nanoparticles with kiwi juice and its novel application for detecting protease K. New J. Chem. 2015, 39, 1295–1300. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.L.; Wang, J.H. Inner filter effect-based fluorescent sensing systems: A review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar] [CrossRef]
- Cao, X.; Wang, J.P.; Deng, W.W.; Chen, J.J.; Wang, Y.; Zhou, J.; Du, P.; Xu, W.Q.; Wang, Q.; Wang, Q.L.; et al. Photoluminescent cationic carbon dots as efficient non-viral delivery of plasmid SOX9 and chondrogenesis of fibroblasts. Sci. Rep. 2018, 8, 7057. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Li, N.; Liu, S.G.; Li, N.B.; Luo, H.Q. A label-free, highly sensitive and selective detection of hemin based on the competition between hemin and protoporphyrin IX binding to G-quadruplexes. Anal. Sci. 2016, 32, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, B.; Zhang, Y.; Zhao, Q.; Zhao, J.; Li, L.; Zuo, G. Acid-treated Graphitic Carbon Nitride Nanosheets as Fluorescence Probe for Detection of Hemin. ChemistrySelect 2019, 4, 8178–8182. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.-T.; Chang, S.; Chen, B.-B.; Li, D.-W. Dual-Exciting Central Carbon Nanoclusters for the Dual-Channel Detection of Hemin. Inorganics 2023, 11, 226. https://doi.org/10.3390/inorganics11060226
Gao Y-T, Chang S, Chen B-B, Li D-W. Dual-Exciting Central Carbon Nanoclusters for the Dual-Channel Detection of Hemin. Inorganics. 2023; 11(6):226. https://doi.org/10.3390/inorganics11060226
Chicago/Turabian StyleGao, Ya-Ting, Shuai Chang, Bin-Bin Chen, and Da-Wei Li. 2023. "Dual-Exciting Central Carbon Nanoclusters for the Dual-Channel Detection of Hemin" Inorganics 11, no. 6: 226. https://doi.org/10.3390/inorganics11060226