Older but Stronger: Development of Platinum-Based Antitumor Agents and Research Advances in Tumor Immunity
Abstract
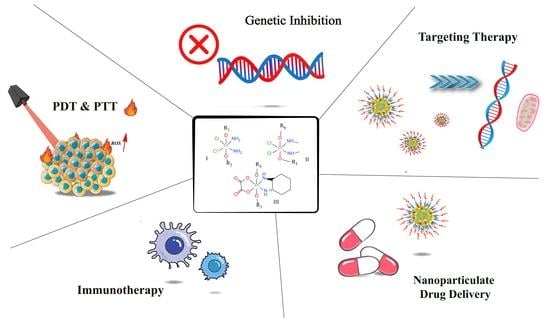
:1. Introduction
2. Multiple Pathways to Enhance Targeting Properties of Pt-Based Antitumor Drugs
2.1. Improving Drug Targeting by Structural Modification of Pt(II) and Pt(IV) Prodrugs
2.2. Nanoparticulate Pt-Based drug Delivery System to Upgrade Intracellular Accumulation
3. Synergistic Involvement of Pt-Based Drugs in Immunotherapy
3.1. Recruiting Immune Cells to Enhance Immunotherapy Effects
3.2. A Promising Combination of ROS and Macrophages to Direct Polarization Strategy
3.3. Combination with Immune Agonists to Improve T-Cell Responses
3.4. Pt-Based Drugs Combined with NSAIDs to Repress Tumor-Related Inflammation
4. Multi-Targeting Structure Modification to Actualize Diverse Antitumor Actions
5. Activation of Pt-Based Prodrugs via Thermal/Invisible Light Stimuli
5.1. NIR-Based Photothermal Therapy Using Pt-Based Drugs
5.2. Pt-Based Drugs for Oxygen-Dependent Photodynamic Therapy
6. Complexes with DNA Expression and Histone Post-Translation Depressants
6.1. Histone Acetylation (HDAC) Inhibitors to Reverse Drug Resistance in Tumor Cells
6.2. DNA Expression Inhibitors of Various Levels to Enlighten Novel Modifications
6.3. Conjugations of Doxorubicin (DOX) and Pt-Based Drugs to Reduce Drug Resistance
7. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| GSH | glutathione |
| ASA | ascorbic acid |
| HIF-1 | hypoxia-inducible factor-1 |
| LA | lactobionic acid |
| EGFR | epidermal growth factor receptor |
| CRGD | carboplatin |
| TDO | tryptophan 2,3-dioxygenase |
| TAM | tumor-associated macrophage |
| TME | tumor immune microenvironment |
| ADCC | antibody-dependent cell-mediated cytotoxicity |
| TREM2 | trigger receptors expressed on myeloid cells 2 |
| MDSC | myeloid-derived suppressor cell |
| ROS | reactive oxygen species |
| MENPs | melanin-like nanoparticles |
| PA | phytic acid |
| PPM | precursor monomer |
| LNP | liposome nanoparticle |
| TLS | translesion synthesis |
| NER | nucleotide excision repair |
| HR | homologous recombination |
| HDAC | histone acetylation |
| HDACi | histone acetylase inhibitors |
| PARP-1 | poly ADP-ribose polymerase-1 |
| DOX | doxorubicin |
| CP6A | carboxylated columnar aromatics |
| 3-ABA | 3-aminobenzamide |
| PDT | photodynamic therapy |
| PTT | photothermal therapy |
| CDT | chemodynamic therapy |
| UVA | ultraviolet-A |
| MRP1 | multidrug resistance-associated protein 1 |
| MPC | 2-methacryloyloxyethyl phosphorylcholine |
| MPS | mononuclear phagocytic system |
References
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Muggia, F.M.; Bonetti, A.; Hoeschele, J.D.; Rozencweig, M.; Howell, S.B. Platinum Antitumor Complexes: 50 Years Since Barnett Rosenberg’s Discovery. JCO 2015, 33, 4219–4226. [Google Scholar] [CrossRef] [PubMed]
- Langer, T.; am Zehnhoff-Dinnesen, A.; Radtke, S.; Meitert, J.; Zolk, O. Understanding Platinum-Induced Ototoxicity. Trends Pharmacol. Sci. 2013, 34, 458–469. [Google Scholar] [CrossRef]
- Zhou, J.; Kang, Y.; Chen, L.; Wang, H.; Liu, J.; Zeng, S.; Yu, L. The Drug-Resistance Mechanisms of Five Platinum-Based Antitumor Agents. Front. Pharmacol. 2020, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Hydes, P.C.; Russell, M.J.H. Advances in Platinum Cancer Chemotherapy: Advances in the Design of Cisplatin Analogues. Cancer Metast. Rev. 1988, 7, 67–89. [Google Scholar] [CrossRef]
- Woloschuk, D.M.M.; Pruemer, J.M.; Cluxton, R.J. Carboplatin: A New Cisplatin Analog. Drug Intell. Clin. Pharm. 1988, 22, 843–849. [Google Scholar] [CrossRef]
- Sutton, E.C.; DeRose, V.J. Early Nucleolar Responses Differentiate Mechanisms of Cell Death Induced by Oxaliplatin and Cisplatin. J. Biol. Chem. 2021, 296, 100633. [Google Scholar] [CrossRef]
- Yambulatov, D.S.; Lutsenko, I.A.; Nikolaevskii, S.A.; Petrov, P.A.; Smolyaninov, I.V.; Malyants, I.K.; Shender, V.O.; Kiskin, M.A.; Sidorov, A.A.; Berberova, N.T.; et al. α-Diimine Cisplatin Derivatives: Synthesis, Structure, Cyclic Voltammetry and Cytotoxicity. Molecules 2022, 27, 8565. [Google Scholar] [CrossRef]
- Romashev, N.F.; Abramov, P.A.; Bakaev, I.V.; Fomenko, I.S.; Samsonenko, D.G.; Novikov, A.S.; Tong, K.K.H.; Ahn, D.; Dorovatovskii, P.V.; Zubavichus, Y.V.; et al. Heteroleptic Pd(II) and Pt(II) Complexes with Redox-Active Ligands: Synthesis, Structure, and Multimodal Anticancer Mechanism. Inorg. Chem. 2022, 61, 2105–2118. [Google Scholar] [CrossRef]
- Achkar, I.W.; Abdulrahman, N.; Al-Sulaiti, H.; Joseph, J.M.; Uddin, S.; Mraiche, F. Cisplatin Based Therapy: The Role of the Mitogen Activated Protein Kinase Signaling Pathway. J. Transl. Med. 2018, 16, 96. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorganic Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Hall, M.D.; Mellor, H.R.; Callaghan, R.; Hambley, T.W. Basis for Design and Development of Platinum(IV) Anticancer Complexes. J. Med. Chem. 2007, 50, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-L.; Qiao, X.; Liu, X.-M.; Song, X.-Q.; Zou, Y.-H.; Li, D.-Q.; Yu, X.-W.; Bao, W.-G.; Xu, J.-Y. Rapid DNA Interstrand Cross-Linking of Pt(IV) Compound. Eur. J. Pharmacol. 2022, 925, 174985. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ding, W.; Zhu, X.; Li, B.; Zeng, F.; Wu, K.; Wu, X.; Wang, F. Ligand Evolution in the Photoactivatable Platinum(IV) Anticancer Prodrugs. Front. Chem. 2022, 10, 876410. [Google Scholar] [CrossRef]
- Chen, J.; Gao, C.; Zhang, Y.; Wang, T.; Qian, Y.; Yang, B.; Dong, P.; Zhang, Y. Inorganic Nano-Targeted Drugs Delivery System and Its Application of Platinum-Based Anticancer Drugs. J. Nanosci. Nanotechnol. 2017, 17, 1–17. [Google Scholar] [CrossRef]
- Rébé, C.; Demontoux, L.; Pilot, T.; Ghiringhelli, F. Platinum Derivatives Effects on Anticancer Immune Response. Biomolecules 2019, 10, 13. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, J.; Gou, S.; Xu, G. Novel Hypoxia-Targeting Pt(iv) Prodrugs. Chem. Commun. 2017, 53, 3749–3752. [Google Scholar] [CrossRef]
- Hu, D.; Yang, C.; Lok, C.-N.; Xing, F.; Lee, P.-Y.; Fung, Y.M.E.; Jiang, H.; Che, C.-M. An Antitumor Bis(N-Heterocyclic Carbene)Platinum(II) Complex That Engages Asparagine Synthetase as an Anticancer Target. Angew. Chem. Int. Ed. 2019, 131, 11030–11034. [Google Scholar] [CrossRef]
- Shevtsov, M.; Huile, G.; Multhoff, G. Membrane Heat Shock Protein 70: A Theranostic Target for Cancer Therapy. Phil. Trans. R. Soc. B 2018, 373, 20160526. [Google Scholar] [CrossRef]
- McKeon, A.M.; Noonan, J.; Devocelle, M.; Murphy, B.M.; Griffith, D.M. Platinum(iv) Oxaliplatin–Peptide Conjugates Targeting MemHsp70+ Phenotype in Colorectal Cancer Cells. Chem. Commun. 2017, 53, 11318–11321. [Google Scholar] [CrossRef]
- Zhong, Y.; Jia, C.; Zhang, X.; Liao, X.; Yang, B.; Cong, Y.; Pu, S.; Gao, C. Synthesis, Characterization, and Antitumor Activity of Novel Tumor-targeted Platinum(IV) Complexes. Appl. Organomet. Chem. 2020, 34, e5577. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Gou, S.; Chen, F. A Trifunctional Pt(II) Complex Alleviates the NHEJ/HR-Related DSBs Repairs to Evade Cisplatin-Resistance in NSCLC. Bioorg. Chem. 2020, 104, 104210. [Google Scholar] [CrossRef]
- Jin, S.; Guo, Y.; Song, D.; Zhu, Z.; Zhang, Z.; Sun, Y.; Yang, T.; Guo, Z.; Wang, X. Targeting Energy Metabolism by a Platinum(IV) Prodrug as an Alternative Pathway for Cancer Suppression. Inorg. Chem. 2019, 58, 6507–6516. [Google Scholar] [CrossRef]
- Liu, X.-M.; Li, Z.; He, X.-R.; Liu, R.-P.; Ma, Z.-Y.; Qiao, X.; Wang, S.-Q.; Xu, J.-Y. Dual-Targeting of the Aromatase Binding Domain of Heme and Androstenedione by Pt(iv) Prodrugs: A New Treatment for Postmenopausal Breast Cancer. Inorg. Chem. Front. 2022, 9, 3470–3483. [Google Scholar] [CrossRef]
- Li, S.; Su, W.; Wu, H.; Yuan, T.; Yuan, C.; Liu, J.; Deng, G.; Gao, X.; Chen, Z.; Bao, Y.; et al. Targeted Tumour Theranostics in Mice via Carbon Quantum Dots Structurally Mimicking Large Amino Acids. Nat. Biomed. Eng. 2020, 4, 704–716. [Google Scholar] [CrossRef]
- Wang, Z. Mesoporous Silica Nanoparticles with Lactose-Mediated Targeting Effect to Deliver Platinum(Iv) Prodrug for Liver Cancer Therapy. J. Mater. Chem. B 2017, 5, 7591–7597. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, Y.; Liu, M.; Jin, D.; Zhang, L.; Cheng, J.; Liu, Y. Conjugation of Oxaliplatin with PEGylated-Nanobody for Enhancing Tumor Targeting and Prolonging Circulation. J. Inorg. Biochem. 2021, 223, 111553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Chen, G.; Gong, S. Carboplatin-Complexed and CRGD-Conjugated Unimolecular Nanoparticles for Targeted Ovarian Cancer Therapy. Macromol. Biosci. 2017, 17, 1600292. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-H.; Pan, C.-H.; Peng, C.-L.; Shieh, M.-J. Panitumumab-Conjugated Pt-Drug Nanomedicine for Enhanced Efficacy of Combination Targeted Chemotherapy against Colorectal Cancer. Adv. Healthc. Mater. 2017, 6, 1700111. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yu, Z.; Das, M.; Huang, L. Nano Codelivery of Oxaliplatin and Folinic Acid Achieves Synergistic Chemo-Immunotherapy with 5-Fluorouracil for Colorectal Cancer and Liver Metastasis. ACS Nano 2020, 14, 5075–5089. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, H.; Wu, M.; Shi, K.; Zhou, C.; Peng, J.; Yang, Q. Targeting Delivery of Lidocaine and Cisplatin by Nanogel Enhances Chemotherapy and Alleviates Metastasis. ACS Appl. Mater. Interfaces 2018, 10, 25228–25240. [Google Scholar] [CrossRef]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma Multiforme (GBM): An Overview of Current Therapies and Mechanisms of Resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, X.; Wang, Q.; Chen, Q.; Lu, Y.; Liu, L.; Zhang, Y.; He, X.; Ruan, C.; Zhang, Y.; et al. Substance P Mediated DGLs Complexing with DACHPt for Targeting Therapy of Glioma. ACS Appl. Mater. Interfaces 2017, 9, 34603–34617. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Iqbal, S.; Du, X.-J.; Yuan, Y.; Yang, X.; Li, H.-J.; Wang, J. Ultrafast Charge-Conversional Nanocarrier for Tumor-Acidity-Activated Targeted Drug Elivery. Biomater. Sci. 2018, 6, 350–355. [Google Scholar] [CrossRef]
- Mitra, R.; Singh, S.; Khar, A. Antitumour Immune Responses. Expert Rev. Mol. Med. 2003, 5, 1–22. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the Tumor Microenvironment in PD-L1/PD-1-Mediated Tumor Immune Escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolušić, E.; Frédérick, R.; De Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.; et al. Reversal of Tumoral Immune Resistance by Inhibition of Tryptophan 2,3-Dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 2497–2502. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Müller, A.; Heseler, K.; Woite, C.; Spekker, K.; MacKenzie, C.R.; Däubener, W. Antimicrobial and Immunoregulatory Properties of Human Tryptophan 2,3-Dioxygenase. Eur. J. Immunol. 2009, 39, 2755–2764. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Chen, F.; Wang, X.; Wang, Y.; Gou, S. Pt(IV) Hybrids Containing a TDO Inhibitor Serve as Potential Anticancer Immunomodulators. J. Inorg. Biochem. 2019, 195, 130–140. [Google Scholar] [CrossRef]
- Yamazaki, T.; Buqué, A.; Ames, T.D.; Galluzzi, L. PT-112 Induces Immunogenic Cell Death and Synergizes with Immune Checkpoint Blockers in Mouse Tumor Models. OncoImmunology 2020, 9, 1721810. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, M.; Milovanovic, M.; Jovanovic, S.; Arsenijevic, N.; Markovic, B.S.; Gazdic, M.; Volarevic, V. In Vitro and in Vivo Anti-Tumor Effects of Selected Platinum(IV) and Dinuclear Platinum(II) Complexes against Lung Cancer Cells. J. Biol. Inorg. Chem. 2017, 22, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, L.; Song, Y.; Li, W.; Xu, L. Targeting Macrophages: A Novel Treatment Strategy in Solid Tumors. J. Transl. Med. 2022, 20, 586. [Google Scholar] [CrossRef] [PubMed]
- Larionova, I.V.; Liu, T.; Riabov, V.; Mossel, D.M.; Patysheva, M.R.; Kiselev, A.M.; Kazakova, E.O.; Cherdyntseva, N.V.; Kzhyshkowska, J. The Clearance of EGF by Tumor-Associated Macrophages Is Suppressed by Chemotherapeutic Agent Cisplatin. Ann. Oncol. 2019, 30, v810. [Google Scholar] [CrossRef]
- Muenchen, H.J.; Aggarwal, S.K.; Misra, H.K.; Andrulis, P.J. Morphological and Histochemical Changes in Macrophage Activity After Novel Anti-Neoplastic Platinum Agents. Microsc. Microanal. 1997, 3, 11–12. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Tang, M.; Li, H.; Guo, X.; Yang, X. The Effects of Umbilical Cord-Derived Macrophage Exosomes Loaded with Cisplatin on the Growth and Drug Resistance of Ovarian Cancer Cells. Drug Dev. Ind. Pharm. 2020, 46, 1150–1162. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, S.; Yuan, H.; Wang, Y.; Cai, L.; Chen, H.; Wang, X.; Song, D.; Wang, X.; Guo, Z.; et al. Platinum-Based TREM2 Inhibitor Suppresses Tumors by Remodeling the Immunosuppressive Microenvironment. Angew. Chem. Int. Ed. 2022, 62, e202213337. [Google Scholar]
- Tian, L.; Shao, M.; Gong, Y.; Wei, T.; Zhu, Y.; Chao, Y.; Liu, Z. Epigenetic Platinum Complexes Breaking the “Eat Me/Don’t Eat Me” Balance for Enhanced Cancer Chemoimmunotherapy. Bioconjugate Chem. 2022, 33, 343–352. [Google Scholar] [CrossRef]
- Shueng, P.-W.; Yu, L.-Y.; Chiu, H.-C.; Chang, H.-C.; Chiu, Y.-L.; Kuo, T.-Y.; Yen, Y.-W.; Lo, C.-L. Early Phago-/Endosomal Escape of Platinum Drugs via ROS-Responsive Micelles for Dual Cancer Chemo/Immunotherapy. Biomaterials 2021, 276, 121012. [Google Scholar] [CrossRef]
- Sica, A.; Porta, C.; Amadori, A.; Pastò, A. Tumor-Associated Myeloid Cells as Guiding Forces of Cancer Cell Stemness. Cancer Immunol. Immunother. 2017, 66, 1025–1036. [Google Scholar] [CrossRef]
- Tang, L.; Cai, D.; Qin, M.; Lu, S.; Hu, M.-H.; Ruan, S.; Jin, G.; Wang, Z. Oxaliplatin-Based Platinum(IV) Prodrug Bearing Toll-like Receptor 7 Agonist for Enhanced Immunochemotherapy. ACS Omega 2020, 5, 726–734. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Karin, M.; Sun, B. Targeting Cancer-Promoting Inflammation—Have Anti-Inflammatory Therapies Come of Age? Nat. Rev. Clin. Oncol. 2021, 18, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Zhang, B.; Wang, Y.; Wang, X.; Gou, S. Design, Synthesis and Biological Evaluation of Antitumor Platinum(II) Agents Conjugated with Non-Steroidal Anti-Inflammatory Drug Species. Bioorg. Chem. 2022, 120, 105633. [Google Scholar] [CrossRef]
- Ju, Z.; Li, M.; Xu, J.; Howell, D.C.; Li, Z.; Chen, F.-E. Recent Development on COX-2 Inhibitors as Promising Anti-Inflammatory Agents: The Past 10 Years. Acta Pharm. Sin. B 2022, 12, 2790–2807. [Google Scholar] [CrossRef]
- Kochel, T.J.; Goloubeva, O.G.; Fulton, A.M. Upregulation of Cyclooxygenase-2/Prostaglandin E 2 (COX-2/PGE 2) Pathway Member Multiple Drug Resistance-Associated Protein 4 (MRP4) and Downregulation of Prostaglandin Transporter (PGT) and 15-Prostaglandin Dehydrogenase (15-PGDH) in Triple-Negative Breast Cancer. Breast Cancer (Auckl) 2016, 10, BCBCR.S38529. [Google Scholar]
- Chen, Y.; Wang, Q.; Li, Z.; Liu, Z.; Zhao, Y.; Zhang, J.; Liu, M.; Wang, Z.; Li, D.; Han, J. Naproxen Platinum(iv) Hybrids Inhibiting Cycloxygenases and Matrix Metalloproteinases and Causing DNA Damage: Synthesis and Biological Evaluation as Antitumor Agents in Vitro and in Vivo. Dalton Trans. 2020, 49, 5192–5204. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Wang, Q.; Li, Z.; Liu, Z.; Hua, X.; Han, J.; Chang, C.; Wang, Z.; Li, D. Albumin-Encapsulated Nanoparticles of Naproxen Platinum(IV) Complexes with Inflammation Inhibitory Competence Displaying Effective Antitumor Activities in Vitro and in Vivo. IJN 2021, 16, 5513–5529. [Google Scholar] [CrossRef]
- Jin, S.; Muhammad, N.; Sun, Y.; Tan, Y.; Yuan, H.; Song, D.; Guo, Z.; Wang, X. Multispecific Platinum(IV) Complex Deters Breast Cancer via Interposing Inflammation and Immunosuppression as an Inhibitor of COX-2 and PD-L1. Angew. Chem. Int. Ed. 2020, 59, 23313–23321. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Q.; Li, L.; Chen, Y.; Cui, J.; Liu, M.; Zhang, N.; Liu, Z.; Han, J.; Wang, Z. Ketoprofen and Loxoprofen Platinum(IV) Complexes Displaying Antimetastatic Activities by Inducing DNA Damage, Inflammation Suppression, and Enhanced Immune Response. J. Med. Chem. 2021, 64, 17920–17935. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Liu, Z.; Wang, Q.; Chen, Y.; Liu, M.; Li, D.; Han, J.; Wang, B. Development of a Series of 4-Hydroxycoumarin Platinum(IV) Hybrids as Antitumor Agents: Synthesis, Biological Evaluation and Action Mechanism Investigation. J. Inorg. Biochem. 2019, 194, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Y.; Li, G.; Liu, Z.; Ma, J.; Liu, M.; Li, D.; Han, J.; Wang, B. Synthesis and Evaluation of Bi-Functional 7-Hydroxycoumarin Platinum(IV) Complexes as Antitumor Agents. Bioorg. Med. Chem. 2019, 27, 2112–2121. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Y.; Luo, Q.; Zhang, Y.; Wu, K.; Wang, F. Multi-Targeted Anticancer Agents. CTMC 2017, 17, 3084–3098. [Google Scholar] [CrossRef]
- Suntharalingam, K.; Wilson, J.J.; Lin, W.; Lippard, S.J. A Dual-Targeting, P53-Independent, Apoptosis-Inducing Platinum(ii) Anticancer Complex, [Pt(BDI QQ)]Cl. Metallomics 2014, 6, 437–443. [Google Scholar] [CrossRef]
- Suntharalingam, K.; Song, Y.; Lippard, S.J. Conjugation of Vitamin E Analog α-TOS to Pt(Iv) Complexes for Dual-Targeting Anticancer Therapy. Chem. Commun. 2014, 50, 2465. [Google Scholar] [CrossRef]
- Liu, F.; Dong, X.; Shi, Q.; Chen, J.; Su, W. Improving the Anticancer Activity of Platinum(iv) Prodrugs Using a Dual-Targeting Strategy with a Dichloroacetate Axial Ligand. RSC Adv. 2019, 9, 22240–22247. [Google Scholar] [CrossRef]
- Hyeraci, M.; Scalcon, V.; Folda, A.; Labella, L.; Marchetti, F.; Samaritani, S.; Rigobello, M.P.; Dalla Via, L. New Platinum(II) Complexes Affecting Different Biomolecular Targets in Resistant Ovarian Carcinoma Cells. ChemMedChem 2021, 16, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shao, J.; Wang, J.; Gong, X.-J.; Liu, W.-X.; Wang, S.; Zhang, Y.; Yang, S.; Zhang, Q.-S.; Wei, J.-X.; et al. Antitumor Effects of New Glycoconjugated Pt II Agents Dual-Targeting GLUT1 and Pgp Proteins. Dalton Trans. 2022, 51, 16082–16092. [Google Scholar] [CrossRef]
- Muth, A.; Kamel, J.; Kaur, N.; Shicora, A.C.; Ayene, I.S.; Gilmour, S.K.; Phanstiel, O. Development of Polyamine Transport Ligands with Improved Metabolic Stability and Selectivity against Specific Human Cancers. J. Med. Chem. 2013, 56, 5819–5828. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Li, Y.; Yue, K.; Li, L.; Xi, Z.; Zhang, X.; Liu, J.; Feng, K.; Ma, Q.; et al. Polyamine-Based Pt(IV) Prodrugs as Substrates for Polyamine Transporters Preferentially Accumulate in Cancer Metastases as DNA and Polyamine Metabolism Dual-Targeted Antimetastatic Agents. J. Med. Chem. 2019, 62, 11324–11334. [Google Scholar] [CrossRef] [PubMed]
- Marin, R. New Opportunities for Light-Based Tumor Treatment with an “Iron Fist” . LightSci. Appl. 2022, 11, 65. [Google Scholar] [CrossRef]
- Ma, L.; Yang, T.; Zhang, Z.; Yin, S.; Song, Z.; Shi, W.; Chu, D.; Zhang, Y.; Zhang, M. Cyanostilbene-Based near-Infrared Emissive Platinum(II) Metallacycles for Cancer Theranostics. Chin. Chem. Lett. 2019, 30, 1942–1946. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Guo, H. Synergic Highly Effective Photothermal-Chemotherapy with Platinum Prodrug Linked Melanin-like Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 356–363. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.; Wei, D.; Zhang, L.; Zhang, X.; Zhang, G.; Ding, D.; Xiao, H.; Zhang, D. A Systematic Strategy of Combinational Blow for Overcoming Cascade Drug Resistance via NIR-Light-Triggered Hyperthermia. Adv. Mater. 2021, 33, 2100599. [Google Scholar] [CrossRef]
- Zhou, Z.; Fan, T.; Yan, Y.; Zhang, S.; Zhou, Y.; Deng, H.; Cai, X.; Xiao, J.; Song, D.; Zhang, Q.; et al. One Stone with Two Birds: Phytic Acid-Capped Platinum Nanoparticles for Targeted Combination Therapy of Bone Tumors. Biomaterials 2019, 194, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Shi, T.; Sun, Y.; You, C.; Wang, S.; Wen, G.; Chen, L.; Zhang, X.; Zhu, J.; Sun, B. Folate-Modified, Indocyanine Green-Loaded Lipid-Polymer Hybrid Nanoparticles for Targeted Delivery of Cisplatin. J. Biomater. Sci. Polym. Ed. 2017, 28, 690–702. [Google Scholar] [CrossRef]
- Yang, G.-G.; Pan, Z.-Y.; Zhang, D.-Y.; Cao, Q.; Ji, L.-N.; Mao, Z.-W. Precisely Assembled Nanoparticles against Cisplatin Resistance via Cancer-Specific Targeting of Mitochondria and Imaging-Guided Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 43444–43455. [Google Scholar] [CrossRef]
- Guo, D.; Xu, S.; Huang, Y.; Jiang, H.; Yasen, W.; Wang, N.; Su, Y.; Qian, J.; Li, J.; Zhang, C.; et al. Platinum(IV) Complex-Based Two-in-One Polyprodrug for a Combinatorial Chemo-Photodynamic Therapy. Biomaterials 2018, 177, 67–77. [Google Scholar] [CrossRef]
- He, Y.; Jin, X.; Guo, S.; Zhao, H.; Liu, Y.; Ju, H. Conjugated Polymer–Ferrocence Nanoparticle as an NIR-II Light Powered Nanoamplifier to Enhance Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 31452–31461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Song, X.; Liang, C.; Yi, X.; Song, G.; Chao, Y.; Yang, Y.; Yang, K.; Feng, L.; Liu, Z. Catalase-Loaded Cisplatin-Prodrug-Constructed Liposomes to Overcome Tumor Hypoxia for Enhanced Chemo-Radiotherapy of Cancer. Biomaterials 2017, 138, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Sun, S.; Sun, R.; Cui, G.; Hong, L.; Rao, B.; Li, A.; Yu, Z.; Kan, Q.; Mao, Z. A Metal–Polyphenol-Coordinated Nanomedicine for Synergistic Cascade Cancer Chemotherapy and Chemodynamic Therapy. Adv. Mater. 2020, 32, 1906024. [Google Scholar] [CrossRef]
- Li, X.; Heyer, W.-D. Homologous Recombination in DNA Repair and DNA Damage Tolerance. Cell Res. 2008, 18, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Basu, A. DNA Damage, Mutagenesis and Cancer. IJMS 2018, 19, 970. [Google Scholar] [CrossRef]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA Damage and the Balance between Survival and Death in Cancer Biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef]
- Audia, J.E.; Campbell, R.M. Histone Modifications and Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019521. [Google Scholar] [CrossRef]
- Jones, P.A.; Issa, J.-P.J.; Baylin, S. Targeting the Cancer Epigenome for Therapy. Nat. Rev. Genet. 2016, 17, 630–641. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, W.; Wang, Z.; Gou, S. Platinum(IV) Prodrugs Multiply Targeting Genomic DNA, Histone Deacetylases and PARP-1. Eur. J. Med. Chem. 2017, 141, 211–220. [Google Scholar] [CrossRef]
- Kelland, L. The Resurgence of Platinum-Based Cancer Chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Karmakar, S.; Poetsch, I.; Kowol, C.R.; Heffeter, P.; Gibson, D. Synthesis and Cytotoxicity of Water-Soluble Dual- and Triple-Action Satraplatin Derivatives: Replacement of Equatorial Chlorides of Satraplatin by Acetates. Inorg. Chem. 2019, 58, 16676–16688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qian, M.; Cui, H.; Zeng, S.; Wang, J.; Chen, Q. Spatiotemporal Concurrent Liberation of Cytotoxins from Dual-Prodrug Nanomedicine for Synergistic Antitumor Therapy. ACS Appl. Mater. Interfaces 2021, 13, 6053–6068. [Google Scholar] [CrossRef]
- Lu, H.; He, S.; Zhang, Q.; Li, X.; Xie, Z.; Wang, Z.; Qi, Y.; Huang, Y. Dual-Sensitive Dual-Prodrug Nanoparticles with Light-Controlled Endo/Lysosomal Escape for Synergistic Photoactivated Chemotherapy. Biomater. Sci. 2021, 9, 7115–7123. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP Inhibitors: Synthetic Lethality in the Clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Slade, D. PARP and PARG Inhibitors in Cancer Treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef]
- LaFargue, C.J.; Dal Molin, G.Z.; Sood, A.K.; Coleman, R.L. Exploring and Comparing Adverse Events between PARP Inhibitors. Lancet Oncol. 2019, 20, e15–e28. [Google Scholar] [CrossRef]
- Gabano, E.; Pinton, G.; Balzano, C.; Boumya, S.; Osella, D.; Moro, L.; Ravera, M. Unsymmetric Cisplatin-Based Pt(IV) Conjugates Containing a PARP-1 Inhibitor Pharmacophore Tested on Malignant Pleural Mesothelioma Cell Lines. Molecules 2021, 26, 4740. [Google Scholar] [CrossRef]
- Oelshlegel, F.J. Glucose-6-Phosphate Dehydrogenase Deficiency in Sickle-Cell Disease. Ann. Intern. Med. 1974, 81, 413. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Meng, Z.; Li, Q.; Huang, X.; Kang, Z.; Dong, H.; Chen, J.; Sun, J.; Dong, Y.; Li, J.; et al. A PH Responsive Complexation-Based Drug Delivery System for Oxaliplatin. Chem. Sci. 2017, 8, 4458–4464. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Meng, Z.; Guo, L.; Yuan, X.; Zhang, Y.; Chai, Y.; Sessler, J.L.; Meng, Q.; Li, C. Supramolecular Combination Chemotherapy: A PH-Responsive Co-Encapsulation Drug Delivery System. Chem. Sci. 2020, 11, 6275–6282. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, B.; Zhang, J.; Li, K.; Liang, Y.; Sun, Y.; Ding, Y.; Han, J. Coordinated PH/Redox Dual-Sensitive and Hepatoma-Targeted Multifunctional Polymeric Micelle System for Stimuli-Triggered Doxorubicin Release: Synthesis, Characterization and in Vitro Evaluation. Int. J. Pharm. 2016, 501, 221–235. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Sun, M.; Cheng, X.; Xu, Y.; Lv, X.; Wang, X.; Tang, R. PH/Redox Dual-Sensitive Platinum (IV)-Based Micelles with Greatly Enhanced Antitumor Effect for Combination Chemotherapy. J. Colloid Interface Sci. 2019, 541, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xiao, J.; Tang, M.; Feng, H.; Chen, W.; Du, M. Platinum Covalent Shell Cross-Linked Micelles Designed to Deliver Doxorubicin for Synergistic Combination Cancer Therapy. IJN 2017, 12, 3697–3710. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Cao, Y.; Hu, B.; Li, T.; Zhang, W.; Zhang, Z.; Gao, J.; Niu, H.; Ding, T.; Wu, J.; et al. Older but Stronger: Development of Platinum-Based Antitumor Agents and Research Advances in Tumor Immunity. Inorganics 2023, 11, 145. https://doi.org/10.3390/inorganics11040145
Liu J, Cao Y, Hu B, Li T, Zhang W, Zhang Z, Gao J, Niu H, Ding T, Wu J, et al. Older but Stronger: Development of Platinum-Based Antitumor Agents and Research Advances in Tumor Immunity. Inorganics. 2023; 11(4):145. https://doi.org/10.3390/inorganics11040145
Chicago/Turabian StyleLiu, Jianing, Yi Cao, Bin Hu, Tao Li, Wei Zhang, Zhongze Zhang, Jinhua Gao, Hanjing Niu, Tengli Ding, Jinzhong Wu, and et al. 2023. "Older but Stronger: Development of Platinum-Based Antitumor Agents and Research Advances in Tumor Immunity" Inorganics 11, no. 4: 145. https://doi.org/10.3390/inorganics11040145