Oxidative Treatments of Pesticides in Rainwater Runoff by HOCl, O3, and O3/H2O2: Effects of pH, Humic Acids and Inorganic Matters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rainwater Sample Collection
2.2. Pesticides and Reagents
2.3. Experimental Procedures
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
3.1. Rainwater Quality Analyses
3.2. Consumption Kinetics of HOCl and O3 in Rainwater Sample
3.3. Effects of Oxidant Dosages
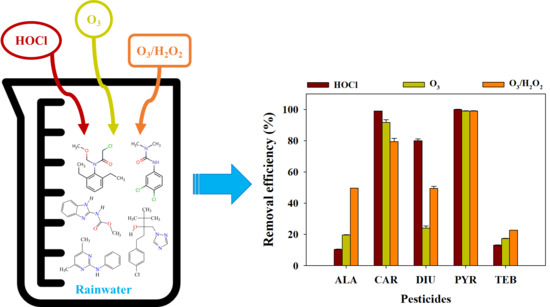
3.4. Removal Efficiency of the Selected Pesticides in Rainwater Samples by HOCl, O3 and O3/H2O2
3.5. Effects of pH on the Removal of the Selected Pesticides
3.6. Effects of Humic Acids on the Removal of the Selected Pesticides
3.7. Effects of Inorganic Matters on the Removal of the Selected Pesticides
4. Conclusions
- DOC is a major rainwater component and has a more significant influence on the consumption kinetics of the oxidants in rainwater than inorganic nitrogen species;
- The dosage of oxidants for the removal of 90% CAR was in the order of HOCl (18 μM) > O3 (21 μM) > O3/H2O2 (25 μM), and the dosage of oxidants for removal of 90% PYR was in the order of O3/H2O2 (9 μM) > O3 (11 μM) > HOCl (20 μM);
- The removal efficiencies of CAR, DIU, and PYR by HOCl were more efficient than those of O3 and O3/H2O2. In contrast, O3/H2O2 was the most effective oxidant for removing ALA and TEB;
- In general, the reactivities of the selected pesticides toward the HOCl, O3, and O3/H2O2 increased due to deprotonation when the pH of the rainwater sample was higher than the pKa values of the selected pesticides;
- The interference effects of HA and inorganic matter in the rainwater on removing the selected pesticides were more significant during the O3/H2O2 process than those of the other oxidation processes;
- These findings suggest that the oxidation processes (i.e., HOCl, O3, and O3/H2O2) might be a promising method to enhance the removal efficiencies of organic pollutants, including pesticides, practically applicable for the wastewater treatment process.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALA | Alachlor |
| C | Concentration of the selected pesticide after oxidation (μmol/L) |
| C0 | Initial concentration of the selected pesticide (μmol/L) |
| CAR | Carbendazim |
| DIU | Diuron |
| DOC | Dissolved organic carbon |
| HA | Humic acids |
| HOCl | Chlorine |
| NO2− | Nitrite |
| NH4+ | Ammonia |
| O3 | Ozone |
| O3/H2O2 | Ozone/hydrogen peroxide |
| pCBA | -chlorobenzoic acid |
| PYR | Pyrimethanil |
| TEB | Tebuconazole |
| TN | Total nitrogen |
References
- Lee, Y.-G.; Shin, J.; Kwak, J.; Kim, S.; Son, C.; Kim, G.-Y.; Lee, C.-H.; Chon, K. Enhanced Adsorption Capacities of Fungicides Using Peanut Shell Biochar via Successive Chemical Modification with KMnO4 and KOH. Separations 2021, 8, 52. [Google Scholar] [CrossRef]
- Grube, A.; Donaldson, D.; Kiely, T.; Wu, L. Pesticides Industry Sales and Usage; US EPA: Washington, DC, USA, 2011. [Google Scholar]
- Rippy, M.A.; Deletic, A.; Black, J.; Aryal, R.; Lampard, J.-L.; Tang, J.Y.-M.; McCarthy, D.; Kolotelo, P.; Sidhu, J.; Gernjak, W. Pesticide occurrence and spatio-temporal variability in urban run-off across Australia. Water Res. 2017, 115, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheyer, A.; Morville, S.; Mirabel, P.; Millet, M. Pesticides analysed in rainwater in Alsace region (Eastern France): Comparison between urban and rural sites. Atmos. Environ. 2007, 41, 7241–7252. [Google Scholar] [CrossRef]
- De Rossi, C.; Bierl, R.; Riefstahl, J. Organic pollutants in precipitation: Monitoring of pesticides and polycyclic aromatic hydrocarbons in the region of Trier (Germany). Phys. Chem. Earth Parts ABC 2003, 28, 307–314. [Google Scholar] [CrossRef]
- Schang, C.; Schmitt, J.; Gao, L.; Bergman, D.; McCormak, T.; Henry, R.; McCarthy, D. Rainwater for residential hot water supply: Managing microbial risks. Sci. Total Environ. 2021, 782, 146889. [Google Scholar] [CrossRef]
- Tran, S.H.; Dang, H.T.; Dao, D.A.; Nguyen, V.-A.; Nguyen, L.T.; Han, M. On-site rainwater harvesting and treatment for drinking water supply: Assessment of cost and technical issues. Environ. Sci. Pollut. Res. 2020, 28, 11928–11941. [Google Scholar] [CrossRef]
- Wang, C.-H.; Blackmore, J.M. Supply–Demand Risk and Resilience Assessment for Household Rainwater Harvesting in Melbourne, Australia. Water Resour. Manag. 2012, 26, 4381–4396. [Google Scholar] [CrossRef]
- Vezzaro, L.; Mikkelsen, P.S. Application of global sensitivity analysis and uncertainty quantification in dynamic modelling of micropollutants in stormwater runoff. Environ. Model. Softw. 2012, 27–28, 40–51. [Google Scholar] [CrossRef]
- Hamers, T.; van den Brink, P.J.; Mos, L.; van der Linden, S.C.; Legler, J.; Koeman, J.H.; Murk, A.J. Estrogenic and esterase-inhibiting potency in rainwater in relation to pesticide concentrations, sampling season and location. Environ. Pollut. 2003, 123, 47–65. [Google Scholar] [CrossRef]
- Nowell, L.H.; Norman, J.E.; Moran, P.W.; Martin, J.D.; Stone, W.W. Pesticide Toxicity Index—A tool for assessing potential toxicity of pesticide mixtures to freshwater aquatic organisms. Sci. Total Environ. 2014, 476–477, 144–157. [Google Scholar] [CrossRef] [Green Version]
- Delpla, I.; Jung, A.V.; Baures, E.; Clement, M.; Thomas, O. Impacts of climate change on surface water quality in relation to drinking water production. Environ. Int. 2009, 35, 1225–1233. [Google Scholar] [CrossRef]
- Gerecke, A.C.; Schärer, M.; Singer, H.P.; Müller, S.R.; Schwarzenbach, R.P.; Sägesser, M.; Ochsenbein, U.; Popow, G. Sources of pesticides in surface waters in Switzerland: Pesticide load through waste water treatment plants––current situation and reduction potential. Chemosphere 2002, 48, 307–315. [Google Scholar] [CrossRef]
- Hamilton, D.; Ambrus, A.; Dieterle, R.; Felsot, A.; Harris, C.; Holland, P.; Katayama, A.; Kurihara, N.; Linders, J.; Unsworth, J. Regulatory limits for pesticide residues in water (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 1123–1155. [Google Scholar] [CrossRef]
- Kudlek, E. Identification of Degradation By-Products of Selected Pesticides During Oxidation and Chlorination Processes. Ecol. Chem. Eng. S 2019, 26, 571–581. [Google Scholar] [CrossRef] [Green Version]
- Wacławek, S. Do We Still Need a Laboratory to Study Advanced Oxidation Processes? A Review of the Modelling of Radical Reactions used for Water Treatment. Ecol. Chem. Eng. S 2021, 28, 11–28. [Google Scholar] [CrossRef]
- Acero, J.L.; Von Gunten, U. Characterization of oxidation processes: Ozonation and the AOPO3/H2O2. J. Am. Water Work. Assoc. 2001, 93, 90–100. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Shin, J.; Kwak, J.; Kim, S.; Son, C.; Cho, K.H.; Chon, K. Effects of NaOH Activation on Adsorptive Removal of Herbicides by Biochars Prepared from Ground Coffee Residues. Energies 2021, 14, 1297. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Park, Y.; Lee, G.; Kim, Y.; Chon, K. Enhanced Degradation of Pharmaceutical Compounds by a Microbubble Ozonation Process: Effects of Temperature, pH, and Humic Acids. Energies 2019, 12, 4373. [Google Scholar] [CrossRef] [Green Version]
- Gibson, K.E.; Schwab, K.J. Tangential-flow ultrafiltration with integrated inhibition detection for recovery of surrogates and human pathogens from large-volume source water and finished drinking water. Appl. Environ. Microbiol. 2011, 77, 385–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.H.; Lee, S.; Kim, Y.M.; Lee, J.H.; Kim, S.K.; Kim, S.G. Pollutants in Rainwater Runoff in Korea: Their Impacts on Rainwater Utilization. Environ. Technol. 2005, 26, 411–420. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yang, J.-S.; Han, M.; Choi, J. Comparison of the microbiological and chemical characterization of harvested rainwater and reservoir water as alternative water resources. Sci. Total Environ. 2010, 408, 896–905. [Google Scholar] [CrossRef]
- Lee, Y.; Von Gunten, U. Oxidative transformation of micropollutants during municipal wastewater treatment: Comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrateVI, and ozone) and non-selective oxidants (hydroxyl radical). Water Res. 2010, 44, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Liu, C.; Dong, B.; Zhang, Y. Degradation mechanism of alachlor during direct ozonation and O3/H2O2 advanced oxidation process. Chemosphere 2010, 78, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Rokbani, O.; Fattouch, S.; Chakir, A.; Roth, E. Heterogeneous oxidation of two triazole pesticides (diniconazole and tebuconazole) by OH-radicals and ozone. Sci. Total Environ. 2019, 694, 133745. [Google Scholar] [CrossRef] [PubMed]
- Mazellier, P.; Leroy, É.; De Laat, J.; Legube, B. Transformation of carbendazim induced by the H2O2/UV system in the presence of hydrogenocarbonate ions: Involvement of the carbonate radical. New J. Chem. 2002, 26, 1784–1790. [Google Scholar] [CrossRef]
- Karaca, H.; Walse, S.S.; Smilanick, J.L. Effect of continuous 0.3μL/L gaseous ozone exposure on fungicide residues on table grape berries. Postharvest Biol. Technol. 2012, 64, 154–159. [Google Scholar] [CrossRef]
- Von Sonntag, C.; Von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment; IWA Publishing: London, UK, 2012. [Google Scholar]
- Acero, J.L.; Real, F.J.; Benitez, F.J.; Gonzalez, M. Kinetics of reactions between chlorine or bromine and the herbicides diuron and isoproturon. J. Chem. Technol. Biotechnol. 2007, 82, 214–222. [Google Scholar] [CrossRef]
- Deborde, M.; Von Gunten, U. Reactions of chlorine with inorganic and organic compounds during water treatment—kinetics and mechanisms: A critical review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]





| Compounds (Abbreviation) | Use | Structure | Molecular Weight a (g/mol) | Solubility in Water a (g/L, pH 7) | UVA Detection (nm) | pKa a | Guideline for Drinking Water (μg/L) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Alachlor (ALA) | Herbicide |  | 269.77 | 0.03 | 210 | - | 20 (WHO) 400 (U.S. EPA) | [14,18] |
| Carbendazim (CAR) | Fungicide |  | 191.19 | 0.43 | 210 | 4.28 | - | [1] |
| Diuron (DIU) | Herbicide |  | 233.09 | 0.18 | 210 | 13.18 | 70 (U.S. EPA) | [14,18] |
| Pyrimethanil (PYR) | Fungicide |  | 199.25 | 0.25 | 210 | 3.44 | - | [1] |
| Tebuconazole (TEB) | Fungicide |  | 307.82 | 0.06 | 210 | 2.01 | - | [1] |
| Para-Chlorobenzoic Acid (pCBA) | OH radical Probe compound |  | 156.57 | 156.57 | 240 | 4.07 | - | [19] |
| Parameters | Conditions |
|---|---|
| pH | 6.6 ± 0.05 |
| Conductivity (μS/cm) | 2.0 ± 0.03 |
| DOC (μgC/L) | 375 ± 1.5 |
| TN (μgN/L) | 70.2 ± 2.0 |
| NO2− (μgN/L) | N.D. |
| NO3− (μgN/L) | 51.3 ± 2.2 |
| Cl− (μg/L) | 198 ± 1.9 |
| SO42− (μg/L) | 485 ± 3.2 |
| PO43− (μg/L) | N.D. |
| NH4+ (μgN/L) | 18.6 ± 1.7 |
| Na+ (μg/L) | 274 ± 7.8 |
| Ca2+ (μg/L) | 206 ± 4.5 |
| Mg2+ (μg/L) | 48.8 ± 8.0 |
| HOCl | O3 | O3/H2O2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pH 5 | pH 7 | pH 9 | pH 5 | pH 7 | pH 9 | pH 5 | pH 7 | pH 9 | |
| ALA | 8.8 ± 0.3 | 10.4 ± 0.2 | 13.5 ± 0.2 | 18.8 ± 0.2 | 19.7 ± 0.1 | 20.6 ± 0.3 | 48.6 ± 0.01 | 49.6 ± 0.03 | 49.9 ± 0.01 |
| CAR | 97.8 ± 1.0 | 99.0 ± 0.01 | 77.6 ± 0.9 | 72.7 ± 1.2 | 91.7 ± 1.8 | 93.0 ± 2.0 | 66.4 ± 2.2 | 79.4 ± 2.1 | 80.5 ± 1.4 |
| DIU | 68.6 ± 1.2 | 80.0 ± 1.2 | 78.6 ± 0.9 | 19.5 ± 1.0 | 24.0 ± 1.4 | 32.1 ± 1.3 | 42.6 ± 1.4 | 49.5 ± 1.2 | 53.0 ± 0.2 |
| PYR | 99.5 ± 0.5 | 99.9 ± 0.2 | 60.7 ± 0.9 | 64.2 ± 1.9 | 99.0 ± 0.1 | 99.1 ± 0.2 | 90.9 ± 1.6 | 99.0 ± 0.2 | 99.5 ± 0.4 |
| TEB | 4.0 ± 0.1 | 13.2 ± 0.2 | 24.4 ± 0.2 | 14.4 ± 0.3 | 17.4 ± 0.2 | 25.5 ± 1.2 | 17.0 ± 1.0 | 22.7 ± 1.4 | 30.3 ± 2.0 |
| HOCl | O3 | O3/H2O2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HA 0 | HA 1 | HA 4 | HA 0 | HA 1 | HA 4 | HA 0 | HA 1 | HA 4 | |
| ALA | 9.5 ± 0.1 | 8.5 ± 0.2 | 5.3 ± 0.2 | 19.1 ± 1.0 | 14.5 ± 0.9 | 12.2 ± 1.0 | 49.0 ± 1.2 | 46.8 ± 0.9 | 34.4 ± 1.6 |
| CAR | 98.2 ± 1.0 | 97.8 ± 1.3 | 67.9 ± 0.9 | 91.7 ± 1.1 | 60.7 ± 1.0 | 10.6 ± 1.2 | 80.7 ± 0.8 | 35.1 ± 1.0 | 3.2 ± 0.1 |
| DIU | 79.2 ± 1.2 | 68.5 ± 1.0 | 45.9 ± 1.0 | 23.8 ± 0.9 | 20.0 ± 1.0 | 16.7± 1.1 | 49.5 ± 1.3 | 19.0 ± 0.9 | 12.1 ± 0.6 |
| PYR | 99.9 ± 0.2 | 99.5 ± 0.1 | 98.1 ± 0.3 | 99.0 ± 0.1 | 98.0 ± 0.8 | 95.3 ± 0.2 | 99.4 ± 0.3 | 74.1 ± 1.2 | 58.9 ± 0.4 |
| TEB | 13.2 ± 1.0 | 9.3 ± 0.6 | 3.7 ± 0.5 | 17.4 ± 1.1 | 12.0 ± 0.9 | 9.6 ± 0.7 | 22.7 ± 1.2 | 15.7 ± 1.0 | 13.6 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochir, D.; Lee, Y.; Shin, J.; Kim, S.; Kwak, J.; Chon, K. Oxidative Treatments of Pesticides in Rainwater Runoff by HOCl, O3, and O3/H2O2: Effects of pH, Humic Acids and Inorganic Matters. Separations 2021, 8, 101. https://doi.org/10.3390/separations8070101
Ochir D, Lee Y, Shin J, Kim S, Kwak J, Chon K. Oxidative Treatments of Pesticides in Rainwater Runoff by HOCl, O3, and O3/H2O2: Effects of pH, Humic Acids and Inorganic Matters. Separations. 2021; 8(7):101. https://doi.org/10.3390/separations8070101
Chicago/Turabian StyleOchir, Duuriimaa, Yonggu Lee, Jaegwan Shin, Sangwon Kim, Jinwoo Kwak, and Kangmin Chon. 2021. "Oxidative Treatments of Pesticides in Rainwater Runoff by HOCl, O3, and O3/H2O2: Effects of pH, Humic Acids and Inorganic Matters" Separations 8, no. 7: 101. https://doi.org/10.3390/separations8070101