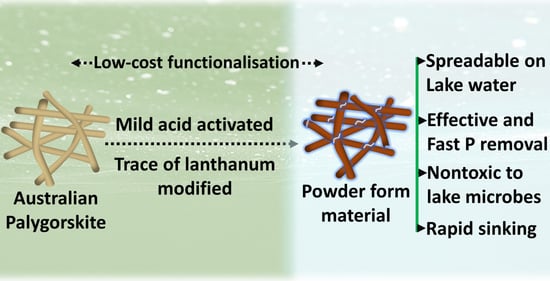
Highly Stable and Nontoxic Lanthanum-Treated Activated Palygorskite for the Removal of Lake Water Phosphorus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. La Modification of Activated Palygorskite
2.3. Characterisation of La-Treated Palygorskite Materials
2.4. Phosphate Adsorption by Modified Clays
2.5. Material Stability, Microbial Toxicity and Settling Capacity of Materials
2.6. Statistical Analysis and Graphical Presentation
3. Results and Discussion
3.1. Characterisation of Materials
3.1.1. Crystalline Phase and Elemental Composition of Modified Materials
3.1.2. La Retention on Palygorskite and Modified Palygorskite
3.1.3. Microscopic Images of Modified Materials and the Elemental State of La
3.2. Removal of Phosphate from Tap and Lake Water
3.3. Material Stability, Binding Mechanism, Biocompatibility and Settling
3.3.1. Leaching of La from the Materials
3.3.2. Potential Binding Mechanism of Phosphate on Materials
3.3.3. Effective Settling and Turbidity of Modified Clays
3.3.4. Microbial Toxicity of Modified Clays
4. Cost-Effective Analysis, Implications and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wigginton, N.S. Fertilizing water contamination. Science 2015, 349, 1297–1298. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Shao, N.; Yang, S.; Ren, H.; Ge, Y.; Feng, P.; Dong, B.; Zhao, Y. Predicting cyanobacteria bloom occurrence in lakes and reservoirs before blooms occur. Sci. Total Environ. 2019, 670, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Moharami, S.; Jalali, M. Use of modified clays for removal of phosphorus from aqueous solutions. Environ. Monit. Assess. 2015, 187, 639. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Lu, S.; Wu, D.; Zhang, Z.; Kong, H. Removal and recovery of phosphate from water by lanthanum hydroxide materials. Chem. Eng. J. 2014, 254, 163–170. [Google Scholar] [CrossRef]
- Razanajatovo, M.R.; Gao, W.; Song, Y.; Zhao, X.; Sun, Q.; Zhang, Q. Selective adsorption of phosphate in water using lanthanum-based nanomaterials: A critical review. Chin. Chem. Lett. 2021, 32, 2637–2647. [Google Scholar] [CrossRef]
- Herrmann, H.; Nolde, J.; Berger, S.; Heise, S. Aquatic ecotoxicity of lanthanum—A review and an attempt to derive water and sediment quality criteria. Ecotoxicol. Environ. Saf. 2016, 124, 213–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Oosterhout, F.; Goitom, E.; Roessink, I.; Lürling, M. Lanthanum from a Modified Clay Used in Eutrophication Control Is Bioavailable to the Marbled Crayfish (Procambarus fallax f. virginalis). PLoS ONE 2014, 9, e102410. [Google Scholar] [CrossRef] [PubMed]
- Spears, B.M.; Lürling, M.; Yasseri, S.; Castro-Castellon, A.T.; Gibbs, M.; Meis, S.; McDonald, C.; McIntosh, J.; Sleep, D.; Van Oosterhout, F. Lake responses following lanthanum-modified bentonite clay (Phoslock®) application: An analysis of water column lanthanum data from 16 case study lakes. Water Res. 2013, 47, 5930–5942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mucci, M.; Douglas, G.; Lürling, M. Lanthanum modified bentonite behaviour and efficiency in adsorbing phosphate in saline waters. Chemosphere 2020, 249, 126131. [Google Scholar] [CrossRef]
- Castro, L.F.d.; Brandão, V.S.; Bertolino, L.C.; Souza, W.F.L.d.; Teixeira, V.G. Phosphate Adsorption by Montmorillonites Modified with Lanthanum/Iron and a Laboratory Test using Water from the Jacarepaguá Lagoon (RJ, Brazil). J. Braz. Chem. Soc. 2019, 30, 641–657. [Google Scholar] [CrossRef]
- Kong, L.; Tian, Y.; Li, N.; Liu, Y.; Zhang, J.; Zhang, J.; Zuo, W. Highly-effective phosphate removal from aqueous solutions by calcined nano-porous palygorskite matrix with embedded lanthanum hydroxide. Appl. Clay Sci. 2018, 162, 507–517. [Google Scholar] [CrossRef]
- Yin, H.; Yang, P.; Kong, M.; Li, W. Use of lanthanum/aluminum co-modified granulated attapulgite clay as a novel phosphorus (P) sorbent to immobilize P and stabilize surface sediment in shallow eutrophic lakes. Chem. Eng. J. 2020, 385, 123395. [Google Scholar] [CrossRef]
- Guggenheim, S.; Krekeler, M.P.S. The Structures and Microtextures of the Palygorskite–Sepiolite Group Minerals. In Developments in Clay Science; Galàn, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 3–32. [Google Scholar]
- Biswas, B.; Sarkar, B.; Naidu, R. Bacterial mineralization of phenanthrene on thermally activated palygorskite: A 14C radiotracer study. Sci. Total Environ. 2017, 579, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Sarkar, B.; Rusmin, R.; Naidu, R. Mild acid and alkali treated clay minerals enhance bioremediation of polycyclic aromatic hydrocarbons in long-term contaminated soil: A 14C-tracer study. Environ. Pollut. 2017, 223, 255–265. [Google Scholar] [CrossRef]
- Melo, D.M.A.; Ruiz, J.A.C.; Melo, M.A.F.; Sobrinho, E.V.; Martinelli, A.E. Preparation and characterization of lanthanum palygorskite clays as acid catalysts. J. Alloy. Compd. 2002, 344, 352–355. [Google Scholar] [CrossRef]
- Rakhimova, N.; Rakhimov, R. Advances in development of calcined clays as supplementary cementitious materials. IOP Conf. Series Mater. Sci. Eng. 2020, 890, 012085. [Google Scholar] [CrossRef]
- Link, D.D.; Walter, P.J.; Kingston, H.M. Development and Validation of the New EPA Microwave-Assisted Leach Method 3051A. Environ. Sci. Technol. 1998, 32, 3628–3632. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- Walton, J.; Wincott, P.; Fairley, N.; Carrick, A. Peak Fitting with CasaXPS: A Casa Pocket Book; Acolyte Science: Salisbury, UK, 2010; p. 132. [Google Scholar]
- Pepper, I.L.; Gerba, C.P. Cultural Methods. In Environmental Microbiology, 2nd ed.; Maier, R.M., Ian, L.P., Charles, P.G., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 173–189. [Google Scholar]
- Torfs, E.; Nopens, I.; Winkler, M.K.H.; Vanrolleghem, P.A.; Balemans, S.; Smets, I.Y. Setling tests. In Experimental Methods in Wastewater Treatment; Loosdrecht, M.C.M.v., Nielsen, P.H., Lopez-Vazquez, C.M., Brdjanovic, D., Eds.; IWA Publishing: London, UK, 2016; pp. 235–262. [Google Scholar]
- Biswas, B.; Sarkar, B.; Naidu, R. Influence of thermally modified palygorskite on the viability of polycyclic aromatic hydrocarbon-degrading bacteria. Appl. Clay Sci. 2016, 134, 153–160. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Li, J.; Chen, D.; Chang, D.; Kong, D.; Frost, R.L. Effect of thermal treatment on adsorption–desorption of ammonia and sulfur dioxide on palygorskite: Change of surface acid–alkali properties. Chem. Eng. J. 2011, 166, 1017–1021. [Google Scholar] [CrossRef]
- España, V.A.A.; Sarkar, B.; Biswas, B.; Rusmin, R.; Naidu, R. Environmental applications of thermally modified and acid activated clay minerals: Current status of the art. Environ. Technol. Innov. 2019, 13, 383–397. [Google Scholar] [CrossRef]
- Dithmer, L.; Lipton, A.; Reitzel, K.; Warner, T.E.; Lundberg, D.; Nielsen, U.G. Characterization of Phosphate Sequestration by a Lanthanum Modified Bentonite Clay: A Solid-State NMR, EXAFS, and PXRD Study. Environ. Sci. Technol. 2015, 49, 4559–4566. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, V.; Bosco, G.E.; Fadini, P.; Mozeto, A.A.; Cestari, A.R.; Carvalho, W.A. Use of a La(III)-modified bentonite for effective phosphate removal from aqueous media. J. Hazard. Mater. 2014, 274, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Xavier, K.C.M.; Santos, M.R.; Oliveira, M.E.R.; Carvalho, M.W.N.C.; Osajima, J.; Filho, E.C.D.S. Effects of acid treatment on the clay palygorskite: XRD, surface area, morphological and chemical composition. Mater. Res. 2014, 17, 3–8. [Google Scholar] [CrossRef]
- García-Sánchez, J.J.; Solache-Ríos, M.; Martínez-Gutiérrez, J.M.; Arteaga-Larios, N.V.; Ojeda-Escamilla, M.C.; Rodríguez-Torres, I. Modified natural magnetite with Al and La ions for the adsorption of fluoride ions from aqueous solutions. J. Fluor. Chem. 2016, 186, 115–124. [Google Scholar] [CrossRef]
- ThermoScientific. Lanthanum. Available online: https://xpssimplified.com/elements/lanthanum.php (accessed on 6 May 2021).
- Li, J.P.H.; Zhou, X.; Pang, Y.; Zhu, L.; Vovk, E.I.; Cong, L.; van Bavel, A.P.; Li, S.; Yang, Y. Understanding of binding energy calibration in XPS of lanthanum oxide by in situ treatment. Phys. Chem. Chem. Phys. 2019, 21, 22351–22358. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Für Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Wang, Y.; Li, X.; Wang, Y. Investigation of phosphate removal mechanisms by a lanthanum hydroxide adsorbent using p-XRD, FTIR and XPS. Appl. Surf. Sci. 2021, 557, 149838. [Google Scholar] [CrossRef]
- Wu, D.; Tian, S.; Long, J.; Peng, S.; Xu, L.; Sun, W.; Chu, H. Remarkable phosphate recovery from wastewater by a novel Ca/Fe composite: Synergistic effects of crystal structure and abundant oxygen-vacancies. Chemosphere 2021, 266, 129102. [Google Scholar] [CrossRef] [PubMed]









| Model | Equation * | Parameters | La@PalU | La@Pal400 | La@Pal3M |
|---|---|---|---|---|---|
| Langmuir | q max (mg/g) | 1.319 | 1.013 | 1.290 | |
| KL (L/mg) | 3.236 | 11.602 | 1.646 | ||
| R2 | 0.907 | 0.350 | 0.977 | ||
| RMSE | 0.099 | 0.180 | 0.0462 | ||
| Freundlich | KF (mg/g) | 0.924 | 0.891 | 0.790 | |
| n (dimensionless) | 5.841 | 20.838 | 4.752 | ||
| R2 | 0.795 | 0.146 | 0.840 | ||
| RMSE | 0.146 | 0.206 | 0.123 | ||
| Langmuir-Freundlich (Sip’s) | q max (mg/g) | 1.227 | 1.002 | 1.243 | |
| KSip | 3.024 | 3.697 | 1.710 | ||
| n (dimensionless) | 2.095 | 24.364 | 1.179 | ||
| R2 | 0.947 | 0.875 | 0.982 | ||
| RMSE | 0.075 | 0.079 | 0.042 |
| Materials | Initial Concentration P (mg/L, Nominal) | Removal Capacity (%) |
|---|---|---|
| La@PalU | 1 | 99.77 ± 0.00 |
| 5 | 41.45 ± 8.26 | |
| 20 | 9.17 ± 0.95 | |
| 50 | 1.46 ± 0.98 | |
| La@Pal400 | 1 | 99.80 ± 0.02 |
| 5 | 40.75 ± 2.00 | |
| 20 | 8.25 ± 0.50 | |
| 50 | 0.38 ± 0.53 | |
| La@Pal3M | 1 | 89.07 ± 2.72 |
| 5 | 47.88 ± 0.80 | |
| 20 | 14.60 ± 0.57 | |
| 50 | 6.68 ± 2.40 |
| Materials | % Loss of Grafted La in | |
|---|---|---|
| MQ Water | Lake Water | |
| La@PalU | 1.03 × 10−4 ± 7.08 × 10−5 | 1.64 × 10−3 ± 7.58 × 10−4 |
| La@Pal400 | 6.55 × 10−4 ± 2.75 × 10−4 | 1.23 × 10−3 ± 1.05 × 10−4 |
| La@Pal3M | 3.94 × 10−3 ± 1.87 × 10−3 | 2.51 × 10−3 ± 1.10 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, B.; Naidu, R. Highly Stable and Nontoxic Lanthanum-Treated Activated Palygorskite for the Removal of Lake Water Phosphorus. Processes 2021, 9, 1960. https://doi.org/10.3390/pr9111960
Biswas B, Naidu R. Highly Stable and Nontoxic Lanthanum-Treated Activated Palygorskite for the Removal of Lake Water Phosphorus. Processes. 2021; 9(11):1960. https://doi.org/10.3390/pr9111960
Chicago/Turabian StyleBiswas, Bhabananda, and Ravi Naidu. 2021. "Highly Stable and Nontoxic Lanthanum-Treated Activated Palygorskite for the Removal of Lake Water Phosphorus" Processes 9, no. 11: 1960. https://doi.org/10.3390/pr9111960