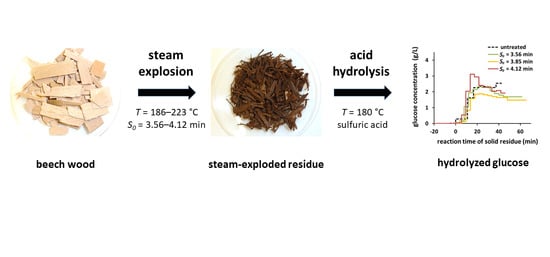
Is Steam Explosion a Promising Pretreatment for Acid Hydrolysis of Lignocellulosic Biomass?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pretreatment via Steam Explosion
2.2. Acid Hydrolysis of Steam-Exploded Residues
2.3. Analytical Methods
3. Results
3.1. Steam Explosion
3.2. Acid Hydrolyisis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Serrano, D.; Coronado, J.; Melero, J. Conversion of cellulose and hemicellulose into platform molecules: Chemical routes. In Biorefinery: From Biomass to Chemicals and Fuels; Aresta, M., Dibenedetto, A., Dumeignil, F., Eds.; De Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Bobleter, O. Hydrothermal degradation of polymers derived from plants. Prog. Polym. Sci. 1994, 19, 797–841. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Steinbach, D.; Kruse, A.; Sauer, J. Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production—A review. Biomass Convers. Biorefinery 2017, 7, 247–274. [Google Scholar] [CrossRef]
- Fan, L.; Gharpuray, M.M.; Lee, Y.H. Cellulose Hydrolysis; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Saini, R.; Osorio-Gonzalez, C.S.; Hegde, K.; Brar, S.K.; Magdouli, S.; Vezina, P.; Avalos-Ramirez, A. Lignocellulosic Biomass-Based Biorefinery: An Insight into Commercialization and Economic Standout. Curr. Sustain. Renew. Energy Rep. 2020. [Google Scholar] [CrossRef]
- Schwald, W.; Breuil, C.; Brownell, H.H.; Chan, M.; Saddler, J.N. Assessment of pretreatment conditions to obtain fast complete hydrolysis on high substrate concentrations. Appl. Biochem. Biotech. 1989, 20–21, 29–44. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X.J. Woody biomass pretreatment for cellulosic ethanol production: Technology and energy consumption evaluation. Bioresour. Technol. 2010, 101, 4992–5002. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. A 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Ferrero, S.; Oriani, L.; Ottonello, P.; Torre, P.; Cherchi, F. Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenergy 2012, 46, 25–35. [Google Scholar] [CrossRef]
- Jacquet, N.; Maniet, G.; Vanderghem, C.; Delvigne, F.; Richel, A. Application of Steam Explosion as Pretreatment on Lignocellulosic Material: A Review. Ind. Eng. Chem. Res. 2015, 54, 2593–2598. [Google Scholar] [CrossRef]
- Alvira, P.; Tomas-Pejo, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Suhag, M.; Dhaka, A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Schultz, T.P.; Templeton, M.C.; Biermann, C.J.; Mcginnis, G.D. Steam Explosion of Mixed Hardwood Chips, Rice Hulls, Corn Stalks, and Sugar-Cane Bagasse. J. Agric. Food Chem. 1984, 32, 1166–1172. [Google Scholar] [CrossRef]
- Jacquet, N.; Vanderghem, C.; Danthine, S.; Quiévy, N.; Blecker, C.; Devaux, J.; Paquot, M. Influence of steam explosion on physicochemical properties and hydrolysis rate of pure cellulose fibers. Bioresour. Technol. 2012, 121, 221–227. [Google Scholar] [CrossRef]
- Carrasco, J.E.; Sáiz, M.C.; Navarro, A.; Soriano, P.; Sáez, F.; Martinez, J.M. Effects of dilute acid and steam explosion pretreatments on the cellulose structure and kinetics of cellulosic fraction hydrolysis by dilute acids in lignocellulosic materials. Appl. Biochem. Biotech. 1994, 45, 23–34. [Google Scholar] [CrossRef]
- Schultz, T.P.; Rughani, J.R.; Mcginnis, G.D. Comparison of the Pretreatment of Sweetgum and White Oak by the Steam Explosion and Rash Processes. Appl. Biochem. Biotech. 1989, 20, 9–27. [Google Scholar] [CrossRef]
- Schultz, T.P.; Biermann, C.J.; Mcginnis, G.D. Steam Explosion of Mixed Hardwood Chips as a Biomass Pretreatment. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 344–348. [Google Scholar] [CrossRef]
- Willfor, S.; Sundberg, A.; Pranovich, A.; Holmbom, B. Polysaccharides in some industrially important hardwood species. Wood Sci. Technol. 2005, 39, 601–617. [Google Scholar] [CrossRef]
- Steinbach, D.; Wüst, D.; Zielonka, S.; Krümpel, J.; Munder, S.; Pagel, M.; Kruse, A. Steam Explosion Conditions Highly Influence the Biogas Yield of Rice Straw. Molecules 2019, 24, 3492. [Google Scholar] [CrossRef] [Green Version]
- Montané, D.; Overend, R.P.; Chornet, E. Kinetic models for non-homogeneous complex systems with a time-dependent rate constant. Can. J. Chem. Eng. 1998, 76, 58–68. [Google Scholar] [CrossRef]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid Hydrolysis of Lignocellulosic Biomass: Sugars and Furfurals Formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef] [Green Version]
- ASTM Standard. Standard Test Method for Acid-Insoluble Lignin in Wood; ASTM Standard: West Conshohocken, PA, USA, 2013; Volume D1106–96. [Google Scholar]
- Saeman, J.F.; Bubl, J.L.; Harris, E.E. Quantitative Saccharification of Wood and Cellulose. Ind. Eng. Chem. Anal. Ed. 1945, 17, 35–37. [Google Scholar] [CrossRef]
- Sawardeker, J.S.; Sloneker, J.H.; Jeanes, A. Quantitative Determination of Monosaccharides as Their Alditol Acetates by Gas Liquid Chromatography. Anal Chem 1965, 37, 1602–1604. [Google Scholar] [CrossRef]
- Negro, M.J.; Manzanares, P.; Oliva, J.M.; Ballesteros, I.; Ballesteros, M. Changes in various physical/chemical parameters of Pinus pinaster wood after steam explosion pretreatment. Biomass Bioenergy 2003, 25, 301–308. [Google Scholar] [CrossRef]
- Springer, E.L.; Harris, J.F. Pre-Hydrolysis of Aspen Wood with Water and with Dilute Aqueous Sulfuric-Acid. Sven. Papp. 1982, 85, 152–154. [Google Scholar]
- Ramos, L.P. The chemistry involved in the steam treatment of lignocellulosic materials. Quim. Nova 2003, 26, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Dinjus, E.; Kruse, A.; Tröger, N. Hydrothermal Carbonization–1. Influence of Lignin in Lignocelluloses. Chem. Eng. Technol. 2011, 34, 2037–2043. [Google Scholar] [CrossRef]
- Titirici, M.M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204–1212. [Google Scholar] [CrossRef] [Green Version]
- Tolonen, L.K.; Zuckerstätter, G.; Penttilä, P.A.; Milacher, W.; Habicht, W.; Serimaa, R.; Kruse, A.; Sixta, H. Structural Changes in Microcrystalline Cellulose in Subcritical Water Treatment. Biomacromolecules 2011, 12, 2544–2551. [Google Scholar] [CrossRef]
- Tolonen, L.K.; Penttilä, P.A.; Serimaa, R.; Kruse, A.; Sixta, H. The swelling and dissolution of cellulose crystallites in subcritical and supercritical water. Cellulose 2013, 20, 2731–2744. [Google Scholar] [CrossRef]





| severity parameter S0 (min) | 3.56 | 3.85 | 4.12 | 4.56 |
| beech wood mass (gdry) | 91.6 | 81.8 | 83.4 | 93.8 |
| steam input time (min) | 25 | 33.5 | 25 | 29 |
| steam input mass (g) | 50 | 80 | 100 | 110 |
| maximum reactor temperature (°C) 1 | 186 | 193 | 206 | 223 |
| maximum excess pressure (bar) 1 | 11 | 13 | 19 | 26 |
| severity parameter S0 (min) | 3.56 | 3.85 | 4.12 | 4.56 |
| solid residue mass (g) | 85.5 | 74.8 | 61.8 | 64.0 |
| solid residue yield (g/gbeech wood) | 0.933 | 0.915 | 0.741 | 0.682 |
| water-insoluble fraction (g/gbeech wood) | 0.789 | 0.724 | 0.612 | 0.533 |
| 0.260 | n.d. | 0.210 | 0.192 |
| 0.103 | n.d. | 0.021 | 0.000 |
| 0.297 | n.d. | 0.353 | 0.346 |
| water-soluble fraction (g/gbeech wood) | 0.144 | 0.191 | 0.128 | 0.149 |
| 0.002 | 0.001 | 0.002 | 0.000 |
| 0.002 | 0.010 | 0.016 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinbach, D.; Kruse, A.; Sauer, J.; Storz, J. Is Steam Explosion a Promising Pretreatment for Acid Hydrolysis of Lignocellulosic Biomass? Processes 2020, 8, 1626. https://doi.org/10.3390/pr8121626
Steinbach D, Kruse A, Sauer J, Storz J. Is Steam Explosion a Promising Pretreatment for Acid Hydrolysis of Lignocellulosic Biomass? Processes. 2020; 8(12):1626. https://doi.org/10.3390/pr8121626
Chicago/Turabian StyleSteinbach, David, Andrea Kruse, Jörg Sauer, and Jonas Storz. 2020. "Is Steam Explosion a Promising Pretreatment for Acid Hydrolysis of Lignocellulosic Biomass?" Processes 8, no. 12: 1626. https://doi.org/10.3390/pr8121626