Control of Solvent-Based Post-Combustion Carbon Capture Process with Optimal Operation Conditions
Abstract
:1. Introduction
2. Process and Simulation
3. Steady-State Optimization
4. Control Schemes and Controller Parameters
5. Results and Discussion
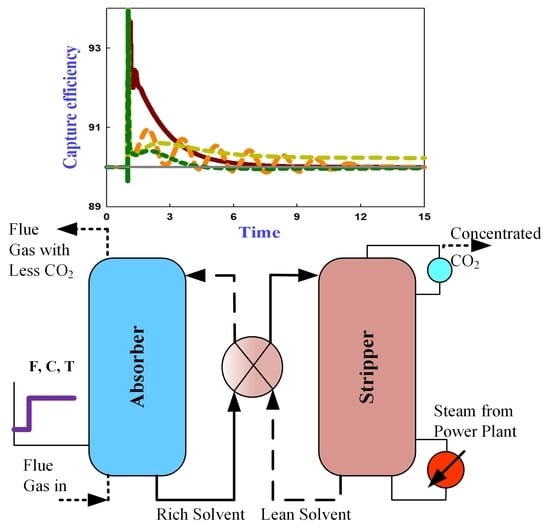
5.1. Dynamic Responses
5.1.1. Disturbance in Flue Gas
- Capture efficiency: For all the control schemes, the capture efficiency sharply dropped to about 85% following the step increase of flue gas flow rate. For all the optimal-based control schemes, the capture efficiency recovers to 90% with obviously less time and less deviation from the target value than the CC- control scheme.
- Reboiler heat duty: The LG-CC control responses to the initial drop of capture efficiency with an initial drastic escalation of reboiler duty because of the CC control loop. On the contrary, LG- and LG--Cascade both responses with an initial drastic decrease of reboiler duty because of the control of , which is quickly raised due to the greater amount of absorption resulted from the higher flue gas flow rate.
- Solvent flow rate: For all the optimal-based control, L/G is controlled and set to its optimal value when the disturbance occurs. On the other hand, the CC- scheme responses with a slow increase in solvent flow rate to a higher than optimal final value.
- Solvent loadings: For all the optimal-based schemes, the response of lean loading is stronger than that of rich loading, while the opposite is true for the CC- scheme. For the CC- scheme, the set-point of stripper temperature is not adjusted with the disturbance change; hence, it results in a smoother change in lean loading. However, the final steady value of lean loading deviates from the optimal value, i.e., 0.24. For all the optimal-based schemes, the new steady state can approach the optimal value.
- Temperatures: Temperature-control related schemes show temperature responses with less damping, expect LG-. In LG-, reboiler heat duty is manipulated to control , and longer response time is needed due to the involvement of many units in the control loop. When the modified scheme, i.e., LG--Cascade, is employed, the temperature responses are much improved.
5.1.2. Capture Efficiency Set-Point Change
- Capture efficiency: The capture efficiency can quickly reach the target value with all the optimal-based control schemes; however, the typical control scheme responds very slowly. Within the optimal-based control schemes, the responses of LG- and LG-CC show more significant damping.
- Reboiler heat duty: In order to obtain fast responses, the optimal-based control schemes require much higher reboiler heat duty with noteworthy damping than the CC- control scheme at the initial period of time.
- Solvent flow rate: For all the optimal-based control, L/G is controlled and set to its optimal value at the same time when the set-point change of the capture efficiency is set. The solvent flow rate of CC- scheme increases very slowly to a higher than optimal final value.
- Solvent loadings: For all the optimal-based schemes, the lean loading responses with more significant damping than the rich loading, while the CC- scheme responds slowly for both lean and rich loadings. For the CC- scheme, the final steady value of lean loading deviates from the optimal value, i.e., 0.24.
- Temperatures: Temperature-control related schemes show temperature responses with less damping, expect LG-. In LG-, as explained above, the long reacting time of the control loop results in a highly damping response. While the modified LG--Cascade scheme provides much better control.
- For all the concentration-related optimal-based control schemes, employing the CC or LL control, the capture efficiency or lean loading can be well controlled.
- For all the temperature-related optimal-based control schemes, except LG-, the temperatures of the absorber, stripper, and rich solvent entering the stripper can be well controlled.
- For all the control schemes, including the optimal-based and the typical, the capture efficiency set-point can be closely approached for disturbance changes and most of the set-point change cases.
- For both disturbance and set-point changes, more significant damping responses are observed for LG- and LG-CC control schemes. The RGA analysis results [34] confirm that inappropriate matches are used by these two schemes. The inferior performance of these two control schemes can also be explained by the longer path between the controlled variable and manipulated variable.
5.2. Stabilization Period Performance
5.3. Effect of Model Error
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| %CC | percent CO2 capture efficiency |
| D | decision variables |
| F | flow rate (kmol/h) |
| G | gas flow rate (kmol/h) |
| IAE | integral of absolute error |
| L | liquid flow rate (kmol/h) |
| LL | lean loading (kmol CO2/kmol MEA) |
| PCC | post-combustion carbon capture |
| Q | heat duty (GJ/hr) |
| specific heat duty (GJ/kmol CO2) | |
| S | process equipment size variables |
| T | temperature (°C) |
| X | process state variables |
Subscripts
| abs | absorber |
| ln | lean solvent |
| mu | makeup |
| MEA | monoethanolamine |
| reb | reboiler |
| rich | rich solvent |
| str | stripper |
References
- The Global Status of CCS: 2018. Available online: https://www.globalccsinstitute.com/resources/global-status-report/ (accessed on 22 December 2018).
- Bui, M.; Gunawan, I.; Verheyen, V.; Feron, P.; Meuleman, E.; Adeloju, S. Dynamic modelling and optimization of flexible operation in post-combustion CO2 capture plants: A review. Comput. Chem. Eng. 2014, 61, 245–265. [Google Scholar] [CrossRef]
- Lawal, A.; Wang, M.; Stephenson, P.; Koumpouras, G.; Yeung, H. Dynamic modelling and analysis of post-combustion CO2 chemical absorption process for coal-fired power plants. Fuel 2010, 89, 2791–2801. [Google Scholar] [CrossRef]
- Panahi, M.; Skogestad, S. Economically efficient operation of CO2 capturing process Part II: Design of control layer. Chem. Eng. Process. 2012, 52, 112–124. [Google Scholar] [CrossRef]
- Moullec, Y.L.; Kanniche, M. Screening of flowsheet modifications for an efficient monoethanolamine (MEA) based post-combustion CO2 capture. Int. J. Greenh. Gas Control 2011, 5, 727–740. [Google Scholar] [CrossRef]
- Moullec, Y.L.; Neveux, T.; Al Azki, A.; Chikukwa, A.; Hoff, K.A. Process modifications for solvent-based post combustion CO2 capture. Energy Procedia 2014, 63, 1470–1477. [Google Scholar] [CrossRef]
- Abu-Zahra, M.R.M.; Schneiders, L.H.J.; Niederer, J.P.M.; Feron, P.H.M.; Versteeg, G.F. CO2 capture from power plants: Part I. A parametric study of the technical performance based on monoethanolamine. Int. J. Greenh. Gas Control 2007, 1, 37–46. [Google Scholar] [CrossRef]
- Abu-Zahra, M.R.M.; Niederer, J.P.M.; Feron, P.H.M.; Versteeg, G.F. CO2 capture from power plants: Part II. A parametric study of the economical performance based on mono-ethanolamine. Int. J. Greenh. Gas Control 2007, 1, 135–142. [Google Scholar] [CrossRef]
- Ziaii, S.; Rochelle, G.T.; Edgar, T.F. Dynamic modeling to minimize energy use for CO2 capture in power plants by aqueous monoethanolamine. Ind. Eng. Chem. Res. 2009, 48, 6105–6111. [Google Scholar] [CrossRef]
- Wellner, K.; Marx-Schubach, T.; Schmitz, G. Dynamic behavior of coal-fired power plants with postcombustion CO2 capture. Ind. Eng. Chem. Res. 2016, 55, 12038–12045. [Google Scholar] [CrossRef]
- Montañés, R.M.; GarÐarsdóttir, S.Ó.; Normann, F.; Johnsson, F.; Nord, L.O. Demonstrating load-change transient performance of a commercial-scale natural gas combined cycle power plant with post-combustion CO2 capture. Int. J. Greenh. Gas Control 2017, 63, 158–174. [Google Scholar] [CrossRef]
- Montañés, R.M.; Flø, N.E.; Nord, L.O. Dynamic process model validation and control of the amine plant at CO2 technology centre mongstad. Energies 2017, 10, 1527. [Google Scholar] [CrossRef]
- Garđarsdóttir, S.Ó.; Montañés, R.M.; Normann, F.; Nord, L.O.; Johnsson, F. Effects of CO2-absorption control strategies on the dynamic performance of a supercritical pulverized-coal-fired power plant. Ind. Eng. Chem. Res. 2017, 56, 4415–4430. [Google Scholar] [CrossRef]
- Martinez Castilla, G.; Biermann, M.; Montañés, R.M.; Normann, F.; Johnsson, F. Integrating carbon capture into an industrial combined-heat-and-power plant: Performance with hourly and seasonal load changes. Int. J. Greenh. Gas Control 2019, 82, 192–203. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Shah, N. MEA-based CO2 capture integrated with natural gas combined cycle or pulverized coal power plants: Operability and controllability through integrated design and control. J. Clean. Prod. 2019, 207, 271–283. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Shah, N. Carbon capture from natural gas combined cycle power plants: Solvent performance comparison at an industrial scale. AIChE 2016, 62, 166–179. [Google Scholar] [CrossRef]
- Panahi, M.; Skogestad, S. Economically efficient operation of CO2 capturing process Part I: Self-optimizing procedure for selecting the best controlled variables. Chem. Eng. Process. 2011, 50, 247–253. [Google Scholar] [CrossRef]
- Nittaya, T.; Douglas, P.L.; Croiset, E.; Ricardez-Sandoval, L.A. Dynamic modelling and control of MEA absorption processes for CO2 capture from power plants. Fuel 2014, 116, 672–691. [Google Scholar] [CrossRef]
- Montañés, R.M.; Flø, N.E.; Nord, L.O. Experimental results of transient testing at the amine plant at Technology Centre Mongstad: Open-loop responses and performance of decentralized control structures for load changes. Int. J. Greenh. Gas Control 2018, 73, 42–59. [Google Scholar] [CrossRef] [Green Version]
- Gjernes, E.; Pedersen, S.; Cents, T.; Watson, G.; Fostås, B.F.; Shah, M.I.; Hamborg, E.S. Results from 30 wt% MEA performance testing at the CO2 technology centre Mongstad. Energy Procedia 2017, 114, 1146–1157. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.-C. Modeling CO2 absorption and desorption by aqueous monoethanolamine solution with Aspen rate-based model. Energy Procedia 2013, 37, 1584–1596. [Google Scholar] [CrossRef]
- Aspen Technology, Inc. Aspen Plus® V10; Aspen Technology, Inc.: Bedford, MA, USA, 2018. [Google Scholar]
- Harun, N.; Nittaya, T.; Douglas, P.L.; Croiset, E.; Ricardez-Sandoval, L.A. Dynamic simulation of MEA absorption process for CO2 capture from power plants. Int. J. Greenh. Gas Control 2012, 10, 95–309. [Google Scholar] [CrossRef]
- Walters, M.S.; Edgar, T.F.; Rochelle, G.T. Dynamic modeling and control of an intercooled absorber for post-combustion CO2 capture. Chem. Eng. Process. 2016, 107, 1–10. [Google Scholar] [CrossRef]
- Flø, N.E.; Knuutila, H.; Kvamsdal, H.M.; Hillestad, M. Dynamic model validation of the post-combustion CO2 absorption process. Int. J. Greenh. Gas Control 2015, 41, 127–141. [Google Scholar]
- Zhang, Y.; Chen, H.; Chen, C.; Plaza, J.M.; Dugas, R.; Rochelle, G.T. Rate-based process modeling study of CO2 capture with aqueous monoethanolamine solution. Ind. Eng. Chem. Res. 2009, 48, 9233–9246. [Google Scholar] [CrossRef]
- Zhang, Q.; Turton, R.; Bhattacharyya, D. Development of model and model-predictive control of an MEA-based post-combustion CO2 capture process. Ind. Eng. Chem. Res. 2016, 55, 1292–1308. [Google Scholar] [CrossRef]
- van de Haar, A.; Trapp, C.; Wellner, K.; De Kler, R.; Schmitz, G.; Colonna, P. Dynamics of post-combustion CO2 capture plants: Modeling, validation, and case study. Ind. Eng. Chem. Res. 2017, 56, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- Chinen, A.S.; Morgan, J.C.; Omell, B.; Bhattacharyya, D.; Miller, D.C. Dynamic data reconciliation and validation of a dynamic model for solvent-based CO2 capture using pilot-plant data. Ind. Eng. Chem. Res. 2019, 58, 1978–1993. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Shen, M.-T.; Chang, H. Dynamic modeling and control of the carbon dioxide capture process using monoethanolamine solvent. J. Appl. Sci. Eng. 2019. paper accepted. [Google Scholar]
- Aspen Technology, Inc. Jump Start: Aspen Custom Modeler® V8; Aspen Technology, Inc.: Bedford, MA, USA, 2015. [Google Scholar]
- Luyben, W.L.; Luyben, M.L. Essentials of Process Control; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Rivera, D.E.; Morari, M.; Skogestad, S. Internal model control 4. PID controller design. Ind. Eng. Chem. Process. Des. Dev. 1986, 25, 252–265. [Google Scholar] [CrossRef]
- Shen, M.-T. Dynamic Simulation and Control of Carbon Dioxide Capture System Using Chemical Absorption. Master’s Thesis, Department of Chemical and Materials Engineering, Tamkang University, New Taipei City, Taiwan, 2018. [Google Scholar]











| Manipulated Variable | Controlled Variable |
|---|---|
| Inventory Control | |
| Absorber rich outflow rate () (V1) | Absorber sump liquid level () |
| Stripper lean outflow rate () (V3) | Stripper sump liquid level () |
| Reflux drum condensate flow rate () (V2) | Reflux drum liquid level () |
| Makeup H2O flow rate () (V4) | Surge tank liquid level () |
| Makeup MEA flow rate () (V5) | Solvent MEA concentration () |
| Quality control | |
| Absorber lean inflow rate () (V6) | CO2 capture efficiency (%CC) |
| Reboiler heat duty () (V7) | Lean loading (LL) |
| Stripper temperature () | |
| Absorber temperature () | |
| Liquid to gas ratio in the absorber (LG) | |
| Controllers and Control Schemes | Gain | Integral Time (min) | Derivative Time (min) |
|---|---|---|---|
| Level controllers for all schemes 1 | 2.0 | - | |
| Flow controllers for CC- 2 | 1.54 | 32.5 | - |
| Flow controllers for other schemes 1 | 0.5 | 0.3 | 0.1 |
| Temperature controllers 2 | |||
| CC- | 0.87 | 17.74 | - |
| LG-CC | 26.59 | 231.81 | - |
| LG-LL | 1.25 | 139.54 | - |
| LG- | 6.17 | 4.66 | - |
| LG- | 1.74 | 62.65 | - |
| LG--Cascade | 74.95 | 143.84 | - |
| Case | LG-CC | LG-LL | LG-Tabs | LG-Tstr | LG-Tabs-Cascade |
|---|---|---|---|---|---|
| +10% change of flue gas flow rate | |||||
| Accurate | −0.05 | −0.12 | −0.02 | −0.18 | 0.03 |
| Error +5% | −0.01 | −1.60 | 0.59 | −1.54 | 0.60 |
| Error −5% | 0.00 | 1.14 | −1.06 | 0.97 | −1.03 |
| −10% change of flue gas flow rate | |||||
| Accurate | 0.04 | 0.16 | 0.00 | 0.23 | −0.03 |
| Error +5% | −1.87 | −1.64 | 0.91 | ||
| Error −5% | 1.79 | −1.06 | 1.73 | −1.07 | |
| +10% change of flue gas concentration | |||||
| Accurate | −0.04 | −0.12 | −0.01 | −0.17 | 0.03 |
| Error +5% | −0.01 | −1.93 | − | −1.85 | 0.67 |
| Error −5% | 0.01 | −1.52 | −1.01 | −1.32 | 0.93 |
| −10% change of flue gas concentration | |||||
| Accurate | 0.01 | 0.12 | 0.01 | 0.17 | −0.03 |
| Error +5% | 0.00 | −1.01 | 0.87 | 1.22 | −0.97 |
| Error −5% | 0.00 | 1.46 | −1.14 | 1.40 | −1.14 |
| +12.5% change of flue gas temperature | |||||
| Accurate | 0.00 | 0.07 | −0.01 | 0.11 | −0.01 |
| Error +5% | 0.00 | −1.71 | 0.85 | −1.53 | 0.85 |
| Error −5% | 0.00 | −1.74 | 0.76 | −1.63 | 0.78 |
| −12.5% change of flue gas temperature | |||||
| Accurate | 0.00 | −0.05 | 0.01 | −0.08 | 0.01 |
| Error +5% | 0.00 | 1.53 | −1.02 | 1.45 | −1.01 |
| Error −5% | 0.00 | 1.36 | −1.11 | 1.22 | −1.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Shen, M.-T.; Chang, H.; Ho, C.-D. Control of Solvent-Based Post-Combustion Carbon Capture Process with Optimal Operation Conditions. Processes 2019, 7, 366. https://doi.org/10.3390/pr7060366
Chen Y-H, Shen M-T, Chang H, Ho C-D. Control of Solvent-Based Post-Combustion Carbon Capture Process with Optimal Operation Conditions. Processes. 2019; 7(6):366. https://doi.org/10.3390/pr7060366
Chicago/Turabian StyleChen, Yih-Hang, Ming-Tien Shen, Hsuan Chang, and Chii-Dong Ho. 2019. "Control of Solvent-Based Post-Combustion Carbon Capture Process with Optimal Operation Conditions" Processes 7, no. 6: 366. https://doi.org/10.3390/pr7060366