A Feasible Route for Preparation of Calcium Sulfate Whiskers from FGD Gypsum via Filtrate Recycle under Hydro-Thermal Conditions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
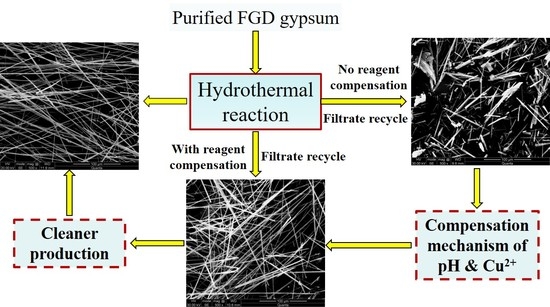
3.1. Effect of Filtrate Recycle Number on Preparation CSWs without Reagent Compensation
3.2. Effect of Filtrate Cycle Number on Preparation CSWs with Reagent Compensation
3.3. Effect of Filtrate Cycle Number on the Productivity of CSWs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, C.J.; Zhao, Q.; Wang, Y.G.; Shi, P.Y.; Jiang, M.F. Surface modification of calcium sulfate whisker prepared from flue gas desulfurization gypsum. Appl. Surf. Sci. 2016, 360, 263–269. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y.; Wang, G.L. Dual-surface modification of calcium sulfate whisker with sodium hexametaphosphate/silica and use as new water-resistant reinforcing fillers in papermaking. Powder Technol. 2015, 271, 1–6. [Google Scholar] [CrossRef]
- Chen, R.Y.; Zou, W.; Wu, C.R.; Jia, S.K.; Huang, Z.G.; Zhang, Z.; Yang, Z.T.; Qu, J.P. Poly(lactic acid)/poly(butylene succinate)/calcium sulfate whiskers biodegradable blends prepared by vane extruder: Analysis of mechanical properties, morphology, and crystallization behavior. Polym. Test. 2014, 34, 1–9. [Google Scholar] [CrossRef]
- Yuan, W.J.; Cui, J.Y.; Cai, Y.B.; Xu, S.A. A novel surface modification for calcium sulfate whisker used for reinforcement of poly(vinyl chloride). J. Polym. Res. 2015, 22, 173–182. [Google Scholar] [CrossRef]
- Han, Q.; Luo, K.B.; Li, H.P.; Xiang, L. Influence of disodium hydrogen phosphate dodecahydrate on hydrothermal formation of hemihydrate calcium sulfate whiskers. Particuology 2014, 17, 131–135. [Google Scholar] [CrossRef]
- He, H.; Dong, F.Q.; He, P.; Xu, L.H. Effect of glycerol on the preparation of phosphogypsum-based CaSO4·0.5H2O whiskers. J. Mater. Sci. 2014, 49, 1957–1963. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Yang, L.S.; Zhu, X.F. Effect of CuCl2 on hydrothermal crystallization of calcium sulfate whiskers prepared from FGD gypsum. Cryst. Res. Technol. 2015, 8, 633–640. [Google Scholar] [CrossRef]
- Xu, A.Y.; Li, H.P.; Luo, K.B.; Xiang, L. Formation of calcium sulfate whiskers from CaCO3-bearing desulfurization gypsum. Res. Chem. Intermed. 2011, 37, 449–455. [Google Scholar] [CrossRef]
- Yuan, Z.T.; Wang, X.L.; Han, Y.X.; Yin, W.Z. Preparation of ultrafine calcium sulfate whiskers by hydrothermal synthesis. J. Northeast. Univ. (Nat. Sci.) 2008, 29, 573–576. [Google Scholar]
- Yuan, Z.T.; Wang, Y.B.; Li, L.X. Preparation integrated with stabilization for hemihydrate calcium sulfate whiskers. J. Northeast. Univ. (Nat. Sci.) 2010, 31, 1636–1639. [Google Scholar]
- Lü, P.F.; Fei, D.J.; Dang, Y.G. Effects of calcium mono hydrogen phosphate on the morphology of calcium sulfate whisker by hydrothermal synthesis. Can. J. Chem. Eng. 2014, 92, 1709–1713. [Google Scholar] [CrossRef]
- Guan, B.H.; Yang, L.C.; Wu, Z.B. Effect of Mg2+ ions on the nucleation kinetics of calcium sulfate in concentrated calcium chloride solution. Ind. Eng. Chem. Res. 2010, 49, 5569–5574. [Google Scholar] [CrossRef]
- Mao, X.L.; Song, X.F.; Lu, G.M.; Sun, Y.Z.; Xu, Y.X.; Yu, J.G. Effects of metal ions on crystal morphology sulfate whiskers in aqueous HCl solutions. Ind. Eng. Chem. Res. 2014, 53, 17625–17635. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.S.; Zhu, X.F.; Yang, J.K. Preparation of calcium sulfate whiskers from FGD gypsum via hydrothermal crystallization in the H2SO4-NaCl-H2O system. Particuology 2014, 17, 42–48. [Google Scholar] [CrossRef]
- Wang, T.X.; Cölfen, H.; Antonietti, M. Nonclassical presence of a polyelectrolyte additive. J. Am. Chem. Soc. 2005, 127, 3246–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldmann, T.; Demopoulos, G.P. Effects of crystal habit modifiers on the morphology of calcium sulfate dihydrate grown in strong CaCl2-HCl solutions. J. Chem. Technol. Biotechnol. 2014, 89, 1523–1533. [Google Scholar] [CrossRef]
- Jones, F.; Ogden, M.I. Controlling crystal growth with modifiers. CrystEngComm 2010, 12, 1016–1023. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, L.S.; Zhu, X.F.; Yang, J.K. Effect of K2SO4/KCl on crystallization of FGD gypsum whisker. J. Synth. Cryst. 2013, 42, 2661–2667. [Google Scholar]
- Mao, X.L.; Song, X.F.; Lu, G.M.; Sun, Y.Z.; Xu, Y.X.; Yu, J.G. Control of crystal morphology and size of calcium sulfate whiskers in aqueous HCl solutions by additives: Experimental and molecular dynamics simulation studies. Ind. Eng. Chem. Res. 2015, 54, 4781–4787. [Google Scholar] [CrossRef]
- Hou, S.; Wang, J.; Wang, X.; Chen, H.; Xiang, L. Effect of Mg2+ on hydrothermal formation of α-CaSO4·0.5H2O whiskers with high aspect ratios. Langmuir 2014, 30, 9804–9810. [Google Scholar] [CrossRef]
- Fu, H.; Huang, J.; Shen, L.; Li, Y.; Xu, L.; Wei, F. Sodium cation-mediated crystallization of α-hemihydrate whiskers from gypsum in ethylene glycol–water solutions. Cryst. Growth Des. 2018, 18, 6694–6701. [Google Scholar] [CrossRef]
- Jia, C.; Chen, Q.; Zhou, X.; Wang, H.; Jiang, G.; Guan, B. Trace NaCl and Na2EDTA mediated synthesis of α-calcium sulfate hemihydrate in glycerol–water solution. Ind. Eng. Chem. Res 2016, 55, 9189–9194. [Google Scholar] [CrossRef]
- Shi, P.Y.; Deng, Z.Y.; Yuan, Y.Y.; Sun, J. Preparation of calcium sulfate whiskers from desulphurized gypsum by hydrothermal synthesis. J. Northeastern. Univ. (Nat. Sci.) 2010, 31, 76–79. [Google Scholar]
- Xin, Y.; Hou, S.C.; Xiang, L.; Yu, Y.X. Adsorption and substitution effects of Mg on the growth of calcium sulfate hemihydrate: An ab initio DFT study. Appl. Surf. Sci. 2015, 357, 1552–1557. [Google Scholar] [CrossRef]
- Liu, T.J.; Fan, H.; Xu, Y.X.; Song, X.F.; Yu, J.G. Effects of metal ions on the morphology of calcium sulfate hemihydrate whiskers by hydrothermal method. Front. Chem. Sci. Eng. 2017, 11, 545–553. [Google Scholar]
- Zhao, W.P.; Gao, C.H.; Zhang, G.Y.; Xu, J.; Wang, C.X.; Wu, Y.M. Controlling the morphology of calcium sulfate hemihydrate using aluminum chloride as a habit modifier. New J. Chem. 2016, 40, 3104–3108. [Google Scholar] [CrossRef]
- Mao, X.L.; Song, X.F.; Lu, G.M.; Xu, Y.X.; Sun, Y.Z.; Yu, J.G. Effect of additives on the morphology of calcium sulfate hemihydrate: Experimental and molecular dynamics simulation studies. Chem. Eng. J. 2015, 278, 320–327. [Google Scholar] [CrossRef]
- Thomas, F.; George, P.D. Influence of impurities on crystallization kinetics of calcium sulfate dihydrate and hemihydrate in strong HCl-CaCl2 solutions. Ind. Eng. Chem. Res. 2013, 52, 6540–6549. [Google Scholar]
- Fu, H.L.; Huang, J.S.; Yin, L.W.; Yu, J.; Lou, W.B.; Jiang, G.M. Retarding effect of impurities on the transformation kinetics of FGD gypsum to alpha-calcium sulfate hemihydrate under atmospheric and hydrothermal conditions. Fuel 2017, 203, 445–451. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Stawski, T.M.; Morgan, D.J.; Peacock, C.L.; Benning, L.G. The effects of inorganic additives on the nucleation and growth kinetics of calcium sulfate dihydratecrystals. Cryst. Growth Des. 2017, 17, 582–589. [Google Scholar] [CrossRef]
- EI-Shall, H.; Rashad, M.M.; Abdel-Aal, E.A. Effect of cetyl pyridinium chloride additive on crystallization of gypsum in phosphoric and sulfuric acids medium. Cryst. Res. Technol. 2005, 40, 860–866. [Google Scholar] [CrossRef]
- Lancia, A.; Musmarra, D.; Prisciandaro, M. Measuring induction period for calcium sulfate dehydrate precipitation. AIChE J. 1999, 45, 390–397. [Google Scholar] [CrossRef]
- Massaro, F.R.; Rubbo, M.; Aquilano, D. Theoretical equilibrium morphology of gypsum(CaSO4·2H2O). 1. A syncretic strategy to calculate the morphology of crystals. Cryst. Growth Des. 2010, 10, 2870–2878. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.S.; Wang, Y.B.; Liu, F.L.; Zhu, X.F. A Purification Technology of FGD Gypsum and the Purified Gypsum. CN Patent 201210112513, 17 April 2012. [Google Scholar]
- Chen, Y.; Yue, W.H.; Dong, R.L. Gypsum Building Materials; China Building Materials Industry Press: Beijing, China, 2003. [Google Scholar]
- Ruan, X.Y.; Peng, M.; Chen, X.M.; Jiang, Z.C. A study on the characteristics of surface pattern and chemical composition variations of fly ash in the solid-phase reaction with H2SO4. J. Instrum. Anal. 2006, 25, 100–102. [Google Scholar]
- Guan, B.H.; Shen, Z.X.; Wu, Z.B.; Yang, L.C.; Ma, X.F. Effect of pH on the preparation of α-calcium sulfate hemihydrate from FGD gypsum with the hydrothermal method. J. Am. Chem. Soc. 2008, 91, 3835–3840. [Google Scholar] [CrossRef]
- Azimi, G.; Papangelakis, V.G. Mechanism and kinetics of gypsum–anhydrite transformation in aqueous electrolyte solutions. Hydrometallurgy 2011, 108, 122–129. [Google Scholar] [CrossRef]
- Dirksen, J.A.; Ring, T.A. Fundamentals of crystallization: Kinetic effects on particle size distributions and morphology. Chem. Eng. Sci. 1991, 46, 2389–2427. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, J. Investigation of the effects of sodium dicarboxylates on the crystal habit of calcium sulfate α-hemihydrate. Langmuir 2017, 33, 9637–9644. [Google Scholar] [CrossRef]
- Guan, Q.J.; Sun, W.; Liu, R.Q.; Yin, Z.G.; Zhang, C.H. Preparation of α-calcium sulfate hemihydrate whiskers with high aspect ratios in presence of a minor amount of CuCl2·2H2O. J. Cent. South Univ. 2018, 25, 526–533. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Wang, Y.B.; Xu, Z.Y.; Zhang, L.; Zhang, X.T.; Yang, L.S. Interaction mechanism of anions in hydrothermal crystallization of desulfurization gypsum whiskers. Chem. J. Chin. Univ. 2021, 41, 473–480. [Google Scholar]
- Wang, X.; Jin, B.; Zhang, X.T.; Zhang, J.W.; Wang, Y.B.; Yuan, D.D.; Yang, L.S. Effect of cation on hydrothermal crystallization of desulfurized gypsum whisker in chloride system and its mechanism. Chem. Ind. Eng. Prog. 2022, 41, 3957–3965. [Google Scholar]
- Cao, B.L.; Wang, X.; Zhang, X.T.; Jin, B.; Xu, Z.Y.; Liu, X.P.; Zhang, W.; Yang, L.S. A readily monitored and controllable hydrothermal system for the facile, cost-effective transformation of FGD gypsum to calcium sulfate hemihydrate whiskers. Particuology 2021, 54, 173–180. [Google Scholar] [CrossRef]







| Sample | Nr-1 | Nr-2 | Nr-3 | Nr-4 | Nr-5 | Nr-6 | Nr-7 | Nr-8 | Nr-9 | Nr-10 |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 3.1 | 2.98 | 3.12 | 3.12 | 3.22 | 3.12 | 3.1 | 3.08 | 3.08 | 3.12 |
| Y1/% | 26.5 | 21.6 | 27.3 | 27.3 | 31.4 | 27.3 | 26.5 | 25.7 | 25.7 | 27.3 |
| Cu2+ concentration/mg·L−1 | 161 | 163 | 159 | 165 | 158 | 160 | 163 | 158 | 164 | 165 |
| Y2/% | 31.8 | 30.9 | 32.5 | 30.1 | 33.1 | 32.2 | 30.9 | 33.1 | 30.5 | 30.1 |
| Composition | SO3 | CaO | SiO2 | Fe2O3 | Al2O3 | MgO | CuO | K2O | Others | |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | Nr-0 | 54.26 | 42.01 | 2.26 | 0.23 | 0.83 | 0.04 | 0.16 | 0.16 | 0.04 |
| Nr-3 | 54.61 | 42.01 | 2.10 | 0.22 | 0.66 | 0.05 | 0.14 | 0.18 | 0.02 | |
| Nr-7 | 54.92 | 42.01 | 1.79 | 0.21 | 0.63 | 0.07 | 0.13 | 0.18 | 0.04 | |
| Nr-10 | 55.00 | 42.40 | 1.78 | 0.16 | 0.38 | 0.04 | 0.09 | 0.14 | 0.01 | |
| Samples | Nr-0 | Nr-1 | Nr-2 | Nr-3 | Nr-4 | Nr-5 | Nr-6 | Nr-7 | Nr-8 | Nr-9 | Nr-10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Productivity (%) | 73.7 | 84.7 | 84.5 | 84.6 | 85.5 | 85.3 | 86.0 | 82.0 | 80.1 | 78.6 | 76.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Jin, B.; Fan, M.; Liu, X.; Zhang, X.; Zhang, J.; Li, S.; Zhang, W. A Feasible Route for Preparation of Calcium Sulfate Whiskers from FGD Gypsum via Filtrate Recycle under Hydro-Thermal Conditions. Processes 2023, 11, 1809. https://doi.org/10.3390/pr11061809
Wang X, Jin B, Fan M, Liu X, Zhang X, Zhang J, Li S, Zhang W. A Feasible Route for Preparation of Calcium Sulfate Whiskers from FGD Gypsum via Filtrate Recycle under Hydro-Thermal Conditions. Processes. 2023; 11(6):1809. https://doi.org/10.3390/pr11061809
Chicago/Turabian StyleWang, Xiao, Biao Jin, Mengke Fan, Xueping Liu, Xiaoting Zhang, Jianwu Zhang, Shanying Li, and Wei Zhang. 2023. "A Feasible Route for Preparation of Calcium Sulfate Whiskers from FGD Gypsum via Filtrate Recycle under Hydro-Thermal Conditions" Processes 11, no. 6: 1809. https://doi.org/10.3390/pr11061809