Experimental Study on CO2 Geochemical Reaction Characteristics in Marine Weakly Consolidated Sandstone Saline Aquifers
Abstract
:1. Introduction
2. Geological Setting
3. Methodology
3.1. Preparation of Samples
- (1)
- Drill cuttings: The drill cuttings in the typical exploration well located at the top of the anticline drilled through aquifers Y and H were used for static CO2 geochemical reaction experiments. Ten typical samples at different depths were selected. Five samples are from aquifer Y, while the other five samples are from aquifer H. There are 2 caprock samples and 3 reservoir samples for each aquifer. The sample number represents the sampling depth of cuttings from shallow to deep, as shown in Table 1.
- (2)
- Sidewall cores: The complete sidewall cores sampled from an exploration well in a nearby block were used for thin section identification before and after the CO2 geochemical reaction. The nearby block has the same sedimentary environment and reservoir physical properties as aquifers Y and H. Twelve typical samples at different depths were selected, of which six samples stand for aquifer Y and the other six samples stand for aquifer H. There are 3 caprock samples and 3 reservoir samples for each aquifer. The sample number also represents the sampling depth of the sidewall cores from shallow to deep and corresponds to the depth of drill cuttings one by one, as shown in Table 1.
- (3)
- Formation water: The real formation waters taken from the aquifers Y and H were used for the CO2 geochemical reaction experiment, with a salinity of 37,806 mg/L and 36,991 mg/L, respectively, and their main ion compositions are similar, as shown in Table 2.
- (4)
- Gas sample: The CO2 content of associated gas in oil reservoir A is up to 95%. After purification, the purity of CO2 will be further improved to larger than 99%; hence, pure CO2 (99.99%) was purchased from Yantai Deyi Gas Co., Ltd. for the experiment.
3.2. Analytical Methods
3.2.1. Static CO2 Geochemical Reaction Experiment
- (1)
- Experimental preparation: (a) The cuttings were cleaned using deionized water to remove the residual drilling mud, dried at 90 °C, and ground into a powder with an average particle size of 200 mesh (45–125 μm) for use. (b) In total, 20 g of cutting powder was taken to analyze the mineral compositions of whole rock and clay minerals using the X-ray diffractometer (XRD) produced by PANalytical B.V. (Model X’Pert PRO MPD, Cu target, and manual mineral identification). (c) In total, 30 g of cutting powder with 150 mL of formation water was put into the reactor, the reactor was sealed with the upper air vacuumed, high-pressure CO2 was injected into the reactor, and the reactor was put into the thermotank for heating; finally, the P and T of the reactor were stabilized at the design values to let the CO2 react with the formation water and cutting powder.
- (2)
- Experimental process: (a) Each set of the CO2 geochemical reaction experiments lasted for 7 days; in the meantime, the reactor was shaken several times to make sure that the bottom cutting powder was fully in contact with the formation water and CO2. (b) The formation water in the reactor was sampled on the 1st, 3rd, 5th, and 7th day, respectively, 5–10 mL at a time, and the contents of K, Na, Ca, Mg, Fe, Al, Si, and pH in the water samples were determined using the inductively coupled plasma mass spectrometer (ICP-MS) produced by Agilent (Model 7500a, the precision is 2.7–4.6%, and the range of error is −3% to 2%).
- (3)
- Experimental analysis: (a) A total of 7 days later, the reactor was opened, the cutting powder after the CO2 geochemical reaction was filtered, dried, and weighed, and 20 g of powder was taken for mineral composition analysis using the XRD method. (b) The CO2 geochemical reaction pathway was analyzed by comparing the compositions of the cutting powder and formation water before and after the CO2 geochemical reaction.
3.2.2. Rock Thin Section Identification before and after CO2 Geochemical Reaction
3.2.3. Numerical Simulation of CO2 Geochemical Reaction
4. Results and Analysis
4.1. Mineral Composition Change Induced by CO2 Geochemical Reaction
4.1.1. Mineral Compositions before CO2 Geochemical Reaction
4.1.2. Mineral Compositions after CO2 Geochemical Reaction
4.2. Rock Micromorphology Change Induced by CO2 Geochemical Reaction
4.2.1. Thin Section Analysis before CO2 Geochemical Reaction
4.2.2. Thin Section Analysis after CO2 Geochemical Reaction
4.3. CO2 Mineral Trapping Capacity by Geochemical Reaction
5. Discussion
6. Conclusions
- (1)
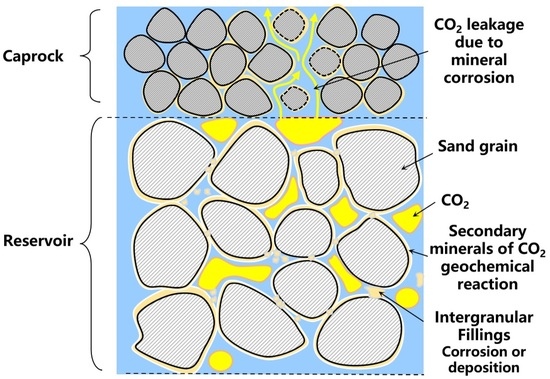
- Saline aquifers Y and H are marine weakly consolidated sandstone formations. The reservoirs are mostly made up of fine-to-medium-grained sandstones as quartz arenite with a considerable amount of feldspar. The detrital grains have a large size, distinct outline, poor sorting, low content of fillings, and large intergranular pores, which can provide favorable pore space for CO2 storage. Comparatively, the caprocks contain a low content of detrital grains with a small size and a high content of argillaceous, siliceous, and ferruginous fillings with a basal cementation and good sorting, which can has a good sealing effect on CO2 storage.
- (2)
- Both the reservoirs and caprocks of aquifers Y and H contain a certain amount of carbonate and clay minerals. When CO2 is injected into aquifers, mineral dissolution will dominate. The contents of quartz and feldspar will increase, while the contents of calcite, dolomite, and clay minerals will decrease. At the same time, more Ca2+ and Mg2+ will be released into the formation water. Due to the different original mineral compositions, the CO2 geochemical reaction is more complicated in caprocks. The specific CO2 geochemical reaction process is determined jointly by the mineral composition, the ion concentrations, the contact surface, the P-T conditions, and the reaction time.
- (3)
- Aquifers Y and H have a theoretical maximum CO2 mineral trapping capacity of 0.023–0.0538 mol/100 g rock, but due to the dynamic equilibrium of the geochemical reaction, only a small number of rock samples can realize the effective utilization of this theoretical capacity, and the largest utilization factor is 75%, while the average utilization factor is only −55.43%. Overall, the amount of mineralized CO2 is negative, and the contribution of mineral trapping to CO2 storage capacity takes −0.32%, which can be ignored.
- (4)
- CO2 geochemical reaction can not only provide the CO2 mineral trapping potential but also change the aquifer’s physical properties and affect the law of CO2 seepage and the safety of CO2 storage. It is very necessary to conduct systematic and detailed research in the future to reveal the effect of geochemical reactions on CO2 storage safety, especially in offshore weakly consolidated sandstone saline aquifers which could be important potential sites for large-scale CO2 storage in China.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, W.Q.; Wang, Q. Key Technologies and prospects for the construction of global energy Internet under the background of carbon neutral. Power Gen. Technol. 2021, 42, 3–7, (In Chinese with English Abstract). [Google Scholar]
- Liu, P.; Yang, W.H.; Zhang, J.; Sun, H.R.; Cui, Z.X. Prospects for emission reduction technologies under carbon neutral targets. Mod. Chem. Ind. 2021, 41, 6–10, (In Chinese with English Abstract). [Google Scholar]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon capture and utilization update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; Coninck, H.D.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: New York, NY, USA, 2005; pp. 51–72. [Google Scholar]
- Damen, K.; Faaij, A.; Turkenburg, W. Health, Safety and environmental risks of underground CO2 storage—Overview of mechanisms and current knowledge. Clim. Chang. 2006, 74, 289–318. [Google Scholar] [CrossRef]
- Zhao, Y.; Rui, Z.H.; Zhang, Z.; Chen, S.W.; Yang, R.F.; Du, K.; Dindoruk, B.; Yang, T.; Stenby, E.H.; Wilson, M.A. Importance of conformance control in reinforcing synergy of CO2 EOR and sequestration. Petrol. Sci. 2022, 19, 3088–3106. [Google Scholar] [CrossRef]
- Ajayi, T.; Gomes, J.S.; Bera, A. A review of CO2 storage in geological formations emphasizing modeling, monitoring and capacity estimation approaches. Petrol. Sci. 2019, 16, 1028–1063. [Google Scholar] [CrossRef]
- Hameli, F.A.; Belhaj, H.; Dhuhoori, M.A. CO2 sequestration overview in geological formations: Trapping mechanisms matrix assessment. Energies 2022, 15, 7805. [Google Scholar] [CrossRef]
- Khan, S.; Al-Shuhail, A.A.; Khulief, Y.A. Numerical modeling of the geomechanical behavior of Ghawar Arab-D carbonate petroleum reservoir undergoing CO2 injection. Environ. Earth Sci. 2016, 75, 1499. [Google Scholar] [CrossRef]
- Newell, P.; Ilgen, A.G. Overview of Geological Carbon Storage (GCS); Report No. SAND2018-3352B662875; Sandia National Lab: Albuquerque, NM, USA, 2018. [Google Scholar]
- Khan, S.; Khulief, Y.A.; Al-Shuhail, A.A. Numerical modeling of the geomechanical behavior of Biyadh reservoir undergoing CO2 injection. Int. J. Geomech. 2017, 17, 04017039. [Google Scholar] [CrossRef]
- Zhang, L. Saline Aquifer Storage of CO2 from Natural Gas Reservoirs in the South China Sea: Trapping Mechanisms and Project Design; China University of Petroleum (East China): Qingdao, China, 2011; (In Chinese with English Abstract). [Google Scholar]
- Zhang, L.; Wen, R.; Geng, S.; Shi, X.; Hao, Y.; Ren, S. Research progress and key issues of CO2 sequestration in basalt minerals. J. Chem. Eng. Chin. Univ. 2022, 36, 473–480, (In Chinese with English Abstract). [Google Scholar]
- Zhang, L.; Wen, R.; Li, F.; Li, C.; Sun, Y. Assessment of CO2 mineral storage potential in the terrestrial basalts of China. Fuel 2023, 037, 157–172. [Google Scholar] [CrossRef]
- Matter, J.M.; Stute, M.; Snæbjornsdottir, S.O.; Oelkers, E.H.; Gislason, S.R.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Sigurdardottir, H.; Gunnlaugsson, E.; et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef]
- McGrail, B.P.; Schaef, H.T.; Ho, A.M.; Chien, Y.J.; Dooley, J.J.; Davidson, C.L. Potential for carbon dioxide sequestration in flood basalts. J. Geophys. Res. 2006, 111, B12201. [Google Scholar] [CrossRef]
- Zou, C.N.; Zhao, W.Z.; Jia, C.Z.; Zhu, R.K.; Zhang, G.Y.; Zhao, X.; Yuan, X.J. Formation and distribution of volcanic hydrocarbon reservoirs in sedimentary basins of China. Petrol. Explor. Dev. 2008, 35, 257–271, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Ren, S.R.; Zhang, L.; Zhang, L. Geological storage of CO2: Overseas demonstration projects and its implications to China. J. Chin. Univ. Petrol. 2010, 34, 93–98, (In Chinese with English Abstract). [Google Scholar]
- Liu, Z.; Zhang, N.; Zhang, F. A review on CO2 geological storage technologies and chemical behaviors of isotope in salty water layer. Sci. Technol. Rev. 2015, 33, 108–113. [Google Scholar]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Bachu, S.; Adams, J.J. Sequestration of CO2 in geological media in response to climate change: Capacity of deep saline aquifers to sequester CO2 in solution. Energ. Convers. Manag. 2003, 44, 3151–3175. [Google Scholar] [CrossRef]
- Czernichowski-Lauriol, I.; Rochelle, C.A.; Brosse, E.; Springer, N.; Bateman, K.; Kervevan, C.; Pearce, J.M.; Sanjuan, B.; Serra, H. Reactivity of Injected CO2 with the Usira Sand Reservoir at Sleipner, Northern North Sea. In Proceedings of the 6th International Conference on Greenhouse Gas Control Technologies, Kyoto, Japan, 1–4 October 2002; pp. 1617–1620. [Google Scholar]
- Gaus, I.; Azaroual, M.; Czernichowski-Lauriol, I. Reactive transport modelling of the impact of CO2 injection on the clayey cap rock at Sleipner (North Sea). Chem. Geol. 2005, 217, 319–337. [Google Scholar] [CrossRef]
- Maldal, T.; Tappel, I.M. CO2 underground storage for Snøhvit gas field development. Energy 2004, 29, 1403–1411. [Google Scholar] [CrossRef]
- Tenthorey, E.; Boreham, C.J.; Hortle, A.L.; Underschultz, J.R.; Golding, S.D. Importance of mineral sequestration during CO2 gas migration: A case study from the Greater Gorgon area. Energy Procedia 2011, 4, 5074–5078. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.F.; Wen, D.G.; Luo, X.W.; Diao, Y.J.; Yang, G.D.; Yin, S.G.; Cao, W. A study of the potential of field-scale of CO2 geological storage and enhanced water recovery in the eastern Junggar area of Xinjiang. Hydrogeol. Eng. Geol. 2021, 48, 196–205, (In Chinese with English Abstract). [Google Scholar]
- Gan, M.G.; Nguyen, M.C.; Zhang, L.W.; Wei, N.; Li, J.; Lei, H.W.; Wang, Y.; Li, X.C.; Stauffer, P.H. Impact of reservoir parameters and wellbore permeability uncertainties on CO2 and brine leakage potential at the Shenhua CO2 Storage Site, China. Int. J. Greenh. Gas Control 2021, 111, 103443. [Google Scholar] [CrossRef]
- He, J.; Yao, Y.; Yu, J.; Zhang, J. Petroleum geological characteristics and exploration and development progress of offshore basins in China. Mar. Geol. Frontier. 2022, 38, 1–17, (In Chinese with English Abstract). [Google Scholar]
- Wang, J.Q.; Yuan, Y.; Chen, J.W.; Zhang, W.; Zhang, J.; Liang, J.; Zhang, Y.G. Geological conditions and suitability evaluation for CO2 geological storage in deep saline aquifers of the Beibu Gulf Basin (South China). Energies 2023, 16, 2360. [Google Scholar] [CrossRef]
- Su, L.; Zheng, J.J.; Chen, G.J.; Zhang, G.C.; Guo, J.M.; Xu, Y.C. The upper limit of maturity of natural gas generation and its implication for the Yacheng formation in the Qiongdongnan Basin, China. J. Asian Earth Sci. 2012, 54–55, 203–213. [Google Scholar] [CrossRef]
- Al-Khdheeawi, E.A.; Mahdi, D.S.; Ali, M.; Fauziah, C.A.; Barifcani, A. Impact of caprock type on geochemical reactivity and mineral trapping efficiency of CO2. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpar, Malaysia, 27 October 2020. [Google Scholar]
- Li, Y.; Tian, H.L.; Zhuo, Z.; Sun, Y. The potential effect of CO2-water-rock reaction on the caprock formation (mudstone) case study. Adv. Mater. Res. 2012, 518–523, 140–143. [Google Scholar] [CrossRef]
- Liu, F.Y.; Lu, P.; Griffith, C.; Hedges, S.W.; Soong, Y.; Hellevang, H.; Zhu, C. CO2-brine-caprock interaction: Reactivity experiments on Eau Claire shale and a review of relevant literature. Int. J. Greenh. Gas Control 2012, 7, 153–167. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2-rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Pearce, J.; Underschultz, J.; Croix, A.L. Mineralogy, Geochemical CO2-Water-Rock Reactions and Associated Characterization; The University of Queensland: Brisbane, Australia, 2019. [Google Scholar]
- Soong, Y.; Crandall, D.; Howard, B.H.; Haljasmaa, I.; Dalton, L.E.; Zhang, L.W.; Lin, R.H.; Dilmore, R.M.; Zhang, W.; Shi, F.; et al. Permeability and mineral composition evolution of primary seal and reservoir rocks in geologic carbon storage conditions. Environ. Eng. Sci. 2018, 35, 391–400. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.Y.; Feng, Y.C.; Qu, L.S.; Liu, J.G.; Li, W.Y.; Dai, M.Y. Damage of reservoir rock induced by CO2 injection. Petrol. Sci. 2022, 19, 1674–1681. [Google Scholar] [CrossRef]
- Gysi, A.P.; Stefánsson, A. Experiments and geochemical modeling of CO2 sequestration during hydrothermal basalt alteration. Chem. Geol. 2012, 306–307, 10–28. [Google Scholar] [CrossRef]
- Pearce, J.K.; Brink, F.; Dawson, G.W.; Poitras, J.; Southam, G.; Paterson, D.J.; Wolhuter, A.; Underschultz, J.R. Core characterisation and predicted CO2 reactivity of sandstones and mudstones from an Australian oil field. Int. J. Coal Geol. 2022, 250, 103911. [Google Scholar] [CrossRef]
- Liu, H.; Ren, Y.L.; Li, X.; Hu, Y.X.; Wu, J.P.; Li, B.; Luo, L.; Tao, Z.; Liu, X.; Liang, J.; et al. Rock thin-section analysis and identification based on artificial intelligent technique. Petrol. Sci. 2022, 19, 1605–1621. [Google Scholar] [CrossRef]
- Ma, H.; Han, G.Q.; Peng, L.; Zhu, L.Y.; Shu, J. Rock thin sections identification based on improved squeeze-and-Excitation Networks model. Comput. Geosci. 2021, 152, 104780. [Google Scholar] [CrossRef]
- Apps, J.A.; Zheng, L.; Spycher, N.; Birkholzer, J.T.; Kharaka, Y.; Thordsen, J.; Kakouros, E.; Trautz, R. Transient changes in shallow groundwater chemistry during the MSU-ZERT CO2 injection experiment. Energy Procedia 2011, 4, 3231–3238. [Google Scholar] [CrossRef]
- CMG. GEM Manual v2021.10; Computer Modelling Group Ltd.: Calgary, AB, Canada, 2021. [Google Scholar]
- Nghiem, L.; Sammon, P.; Grabenstetter, J.; Ohkuma, H. Modeling CO2 Storage in Aquifers with a Fully-Coupled Geochemical EOS Compositional Simulator. In Proceedings of the SPE-89474, SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 17 April 2004. [Google Scholar]
- Ji, L.; Zhang, T.; Milliken, K.; Qu, J.; Zhang, X. Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 2012, 27, 2533–2545. [Google Scholar] [CrossRef]
- Garcia, J.E. Fluid Dynamics of Carbon Dioxide Disposal into Saline Aquifers. University of California: Berkeley, CA, USA, 2003. [Google Scholar]
- Wu, D.S. Fundamentals of Chemical Engineering; Higher Education Press: Beijing, China, 2000; Volume 1. (In Chinese) [Google Scholar]
- Cui, G.D.; Zhu, L.H.; Zhou, Q.C.; Ren, S.R.; Wang, J.Y. Geochemical reactions and their effect on CO2 storage efficiency during the whole process of CO2 EOR and subsequent storage. Int. J. Greenh. Gas Control 2021, 108, 103335. [Google Scholar] [CrossRef]
- Alemu, B.L.; Aagaard, P.; Munz, I.A.; Skurtveit, E. Caprock interaction with CO2: A laboratory study of reactivity of shale with supercritical CO2 and brine. Appl. Geochem. 2011, 26, 1975–1989. [Google Scholar] [CrossRef]
- Wollenweber, J.; Alles, S.; Busch, A.; Krooss, B.M.; Stanjek, H.; Littke, R. Experimental investigation of the CO2 sealing efficiency of caprocks. Int. J. Greenh. Gas Control 2010, 4, 231–241. [Google Scholar] [CrossRef]
- Gherardi, F.; Xu, T.; Pruess, K. Numerical modeling of self-limiting and self-enhancing caprock alteration induced by CO2 storage in a depleted gas reservoir. Chem. Geol. 2007, 244, 103–129. [Google Scholar] [CrossRef]
- Luquot, L.; Gouze, P. Experimental determination of porosity and permeability changes induced by injection of CO2 into carbonate rocks. Chem. Geol. 2009, 265, 148–159. [Google Scholar] [CrossRef]
- Kim, K.; Vilarrasa, V.; Makhnenko, R.Y. CO2 injection effect on geomechanical and flow properties of calcite-rich reservoirs. Fluids 2018, 3, 66. [Google Scholar] [CrossRef]
- Yang, G.; Ma, X.; Feng, T.; Yu, Y.; Yin, S.; Huang, M.; Wang, Y. Geochemical modelling of the evolution of caprock sealing capacity at the Shenhua CCS Demonstration Project. Minerals 2020, 10, 1009. [Google Scholar] [CrossRef]
- Huo, C.L. Study on Potential Evaluation and the Storage Areas of the Carbon Dioxide Seabed Storage in Offshore China; Dalian Maritime University: Dalian, China, 2014. [Google Scholar]













| Aquifers | Formations | Drill Cuttings | Sidewall Cores | ||
|---|---|---|---|---|---|
| No. | Description | No. | Description | ||
| Aquifer Y | Caprock | 1 | Upper caprock | 1 | Upper caprock |
| / | / | 2 | Middle caprock | ||
| 2 | Lower caprock | 3 | Lower caprock | ||
| Reservoir | 3 | Upper, poor physical properties | 4 | Upper, poor physical properties | |
| 4 | Middle, medium physical properties | 5 | Middle, good physical properties | ||
| 5 | Lower, good physical properties | 6 | Lower, medium physical properties | ||
| Aquifer H | Caprock | 6 | Upper caprock | 7 | Upper caprock |
| / | / | 8 | Middle caprock | ||
| 7 | Lower caprock | 9 | Lower caprock | ||
| Reservoir | 8 | Upper, good physical properties | 10 | Upper, good physical properties | |
| 9 | Middle, mudstone interlayer | 11 | Middle, poor physical properties | ||
| 10 | Lower, medium physical properties | 12 | Lower, medium physical properties | ||
| Ion Contents, mg/L | Aquifer Y | Aquifer H |
|---|---|---|
| K+ | 1455 | 1078 |
| Na+ | 12,807 | 12,799 |
| Ca2+ | 494 | 512 |
| Mg2+ | 214 | 192 |
| Fe2+ | 0.31 | 0.14 |
| Fe3+ | 0.65 | 0.57 |
| Al3+ | 1.07 | 3.88 |
| Ba2+ | 1.39 | 3.57 |
| Cl− | 22,163 | 21,670 |
| SO42- | 289 | 198 |
| HCO3- | 338 | 457 |
| CO32- | 0.0 | 0.0 |
| SiO2 | 35.7 | 69.1 |
| Salinity | 37,806 | 36,991 |
| Free CO2 | 30.30 | 28.4 |
| pH | 6.99 | 7.15 |
| Water type | CaCl2 | CaCl2 |
| Aquifer | Formation | T, °C | P, MPa | Reaction Time, Day | Static Geochemical Reaction Experiment | Thin Section Identification | ||
|---|---|---|---|---|---|---|---|---|
| Number, Set | Rock Sample | Number, Set | Rock Sample | |||||
| Aquifer Y | Caprock | 52.4 | 8.39 | 7 | 2 | Drill cuttings | 3 | Sidewall cores |
| Reservoir | 52.4 | 8.39 | 7 | 3 | Drill cuttings | 3 | Sidewall cores | |
| Aquifer H | Caprock | 66.9 | 11.64 | 7 | 2 | Drill cuttings | 3 | Sidewall cores |
| Reservoir | 66.9 | 11.64 | 7 | 3 | Drill cuttings | 3 | Sidewall cores | |
| Total | 10 | 12 | ||||||
| No. | Reaction Equation | lgKeqa(50 °C) |
|---|---|---|
| 1 | H2O = H+ + OH- | −13.2631 |
| 2 | CO2(aq) + H2O = H+ + HCO3- | −6.3221 |
| 3 | CO2(aq) + H2O = 2H+ + CO32- | −16.5563 |
| No. | Mineral | Reaction Equation | Equilibrium Constant lgKeq = a0 + a1 × t + a2 × t2 + a3 × t3 + a4 × t4, WHere, t Is Temperature, °C | Equilibrium Constant lgKeq@52.4 °C | Equilibrium Constant lgKeq@66.9 °C | Activation Energy, J/mol | Reaction Frequency Factor, mol/m2/s1 @25 ℃ | Reaction Surface Area, m2/m3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a0 | a1 | a2 | a3 | a4 | ||||||||
| 1 | Quartz SiO2 | Quartz = SiO2(aq) | −4.4970 | 2.1973 × 10−2 | −1.0732 × 10−4 | 3.2461 × 10−7 | −3.9670 × 10−10 | −3.5965 | −3.4180 | 87,500 | −13.9 | 7182 |
| 2 | K−feldspar KAlSi3O8 | K−feldspar + 4H+ = 2H2O + K+ + Al3+ + 3SiO2(aq) | 4.6130 × 10−1 | −1.5114 × 10−2 | −3.8920 × 10−5 | 4.2895 × 10−7 | −9.2187 × 10−10 | −3.8279 × 10−1 | −6.1408 × 10−1 | 67,830 | −12 | 176 |
| 3 | Albite NaAlSi3O8 | Albite + 4H+ = 2H2O + Na+ + Al3+ + 3SiO2(aq) | 3.9232 | −3.4500 × 10−2 | 2.5244 × 10−5 | 3.0994 × 10−7 | −8.3137 × 10−10 | 2.2230 | 1.8042 | 67,830 | −12 | 250 |
| 4 | Anorthite Ca [Al2Si2O8] | Anorthite + 8H+ = 4H2O + Ca2+ + 2Al3 + + 2SiO2(aq) | 3.1746 × 10 | −2.0125 × 10−1 | 5.9589 × 10−4 | −9.0412 × 10−7 | 9.1539 × 10−11 | 2.2707 × 10 | 2.0680 × 10 | 67,830 | −12 | 88 |
| 5 | Calcite CaCO3 | Calcite + H+ = Ca2+ + HCO3− | 2.0689 | −1.4267 × 10−2 | −6.0610 × 10−6 | 1.4592 × 10−7 | −4.1893 × 10−10 | 1.3225 | 1.1226 | 41,870 | −8.8 | 88 |
| 6 | Dolomite CaMg(CO3)2 | Dolomite + 2H+ = Ca2+ + Mg2+ + 2HCO3− | 3.3944 | −3.5598 × 10−2 | 1.3261 × 10−5 | 2.4106 × 10−7 | −8.1493 × 10−10 | 1.5940 | 1.1281 | 41,870 | −9.2218 | 88 |
| 7 | Ankerite CaMg0.3Fe0.7(CO3)2 | Ankerite + 2H+ = Ca2+ + 0.3Mg2+ + 2HCO3− + 0.7Fe2+ | 4.4773 × 10−1 | −2.5961 × 10−2 | 3.3916 × 10−7 | 8.9790 × 10−8 | −3.4126 × 10−10 | −9.0135 × 10−1 | −1.2675 | 46,276 | −8.8 | 88 |
| 8 | Siderite FeCO3 | Siderite + H+ = HCO3− + Fe2+ | 2.5397 × 10−1 | −1.9361 × 10−2 | 9.4810 × 10−6 | 1.1710 × 10−7 | −4.1245 × 10−10 | −7.2077 × 10−1 | −9.7204 × 10−1 | 41,870 | −9.22 | 88 |
| 9 | Pyrite FeS2 | Pyrite +H2O = Fe2+ + 0.25H+ + 0.25SO42− + 1.75HS− | −2.6441 × 10 | 7.6600 × 10−2 | −2.8402 × 10−4 | 4.6293 × 10−7 | −3.9254 × 10−10 | −2.3143 × 10 | −2.2457 × 10 | 56.9 | −4.55C | 50.1 |
| 10 | Illite K0.6Mg0.25Al1.8(Al0.5Si3.5O10)(OH)2 | Illite + 8H+ = 5H2O + 0.6K+ + 0.25Mg2+ + 2.3Al3+ + 3.5 SiO2(aq) | 1.2435 × 10 | −1.1227 × 10−1 | 2.7171 × 10−4 | −8.0564 × 10−8 | −8.3378 × 10−10 | 7.2802 | 6.0994 | 58,620 | −14 | 8573 |
| 11 | Chlorite Fe2.5Mg2.5Al2Si3O10(OH)8 | Chlorite + 16H+ = 2.5Fe2+ +2.5 Mg2+ + 12H2O+ 2Al3+ + 3SiO2(aq) | 6.6571 | −1.0170 × 10−1 | 2.0000 × 10−4 | −2.0000 × 10−7 | −5.0000 × 10−10 | 1.8446 | 6.7859 × 10−1 | 62,760 | −14 | 14,178 |
| 12 | Kaolinite Al2Si2O5(OH)4 | Kaolinite + 6H+ = 5H2O + 2Al3+ + 2SiO2(aq) | 9.7295 | −9.8898 × 10−2 | 2.9156 × 10−4 | −3.2703 × 10−7 | −3.3110 × 10−10 | 5.2983 | 4.3136 | 62,760 | −13 | 17,600 |
| 13 | Smectite Ca0.145Mg0.26Al1.77Si3.97O10(OH)2 | Smectite + 6.12H+ = 0.145Ca2+ + 0.26Mg2+ + 1.77Al3+ + 3.97SiO2(aq) + 4.06H2O | 2.3304 × 10 | −1.2959 × 10−1 | 4.6020 × 10−4 | −1.0434 × 10−6 | 8.4942 × 10−10 | 1.7633 × 10 | 1.6399 × 10 | 58,620 | −14 | 26,400 |
| Sample # | Mineral Composition of Whole Rock, % | Composition of Clay Minerals, % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartz | K-FELDSPAR | Plagioclase | Calcite | Dolomite | Ankerite | Siderite | Pyrite | Clay Minerals | Kaolinite | Chlorite | Illite | Illite/Smectite | |
| 1 | 60.86 | 6.03 | 8.5 | 3.84 | - | 8.42 | - | 1.83 | 10.52 | 17 | 23 | 33 | 27 |
| 2 | 82.37 | 6.06 | 3.76 | 1.58 | - | 1.26 | - | 0.77 | 4.2 | 20 | 25 | 34 | 21 |
| 3 | 76.97 | 5.16 | 5.67 | 1.95 | - | 1.52 | - | 1.03 | 7.7 | 19 | 24 | 34 | 23 |
| 4 | 74.39 | 5.99 | 6.25 | 4.93 | 1.17 | 1.05 | - | 0.76 | 5.46 | 22 | 22 | 34 | 22 |
| 5 | 66.43 | 5.46 | 6.21 | 6.74 | 1.28 | 0.9 | - | 1.47 | 11.51 | 20 | 24 | 36 | 20 |
| 6 | 67.7 | 6.98 | 6.08 | 7 | - | 1.84 | 1.2 | 1.45 | 7.75 | 22 | 20 | 33 | 25 |
| 7 | 68.14 | 7.24 | 6.32 | - | 1.47 | - | 0.98 | 1.71 | 14.14 | 25 | 17 | 33 | 25 |
| 8 | 63.54 | 6.63 | 6.31 | 6.63 | - | 1.5 | - | 1.06 | 12.78 | 24 | 19 | 34 | 23 |
| 9 | 63.59 | 7.85 | 6.36 | 5.55 | 0.79 | 0.79 | 0.9 | 0.91 | 12.16 | 20 | 19 | 32 | 29 |
| 10 | 68.28 | 7.67 | 6.12 | 7.06 | - | 1.15 | - | 0.72 | 9 | 23 | 20 | 33 | 24 |
| Sample # | Mineral Composition of Whole Rock, % | Composition of Clay Minerals, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartz | K-Feldspar | Plagioclase | Calcite | Ankerite | Siderite | Pyrite | Clay Minerals | Kaolinite | Chlorite | Illite | Illite/Smectite | |
| 1 | 61.73 | 6.01 | 7.18 | 4.37 | 7.41 | - | 2.42 | 10.88 | 21 | 21 | 30 | 28 |
| 2 | 85 | 5 | 4.29 | 1.28 | 1.04 | - | 0.5 | 2.89 | 22 | 22 | 31 | 25 |
| 3 | 78.79 | 5.38 | 6.41 | 1.57 | 1.52 | - | 0.9 | 5.43 | 18 | 18 | 33 | 31 |
| 4 | 75.87 | 6.18 | 6.16 | 3.73 | 1.24 | - | 0.9 | 5.92 | 20 | 19 | 29 | 32 |
| 5 | 67.64 | 6.12 | 8.55 | 4.5 | 1.95 | - | 1.08 | 10.16 | 20 | 18 | 29 | 33 |
| 6 | 68.7 | 8.52 | 6.59 | 6.23 | 1.57 | 0.9 | 1.41 | 6.08 | 20 | 16 | 23 | 41 |
| 7 | 69.78 | 7.48 | 6.94 | - | 1.12 | - | 1.42 | 13.26 | 21 | 14 | 22 | 43 |
| 8 | 61.6 | 7.54 | 7.65 | 6.75 | 1.52 | 1.11 | 1.19 | 12.64 | 18 | 15 | 29 | 38 |
| 9 | 68.04 | 7.1 | 5.46 | 4.28 | 3.48 | 0.6 | 1.5 | 9.54 | 20 | 18 | 27 | 35 |
| 10 | 69.93 | 6.59 | 4.69 | 6.46 | 0.88 | 0.71 | 0.78 | 9.96 | 20 | 17 | 30 | 33 |
| No. | Amount of Mineralized CO2, Mol/100 g Rock | Finial Utilization Factor, % | |||||
|---|---|---|---|---|---|---|---|
| Theoretical Max. Value | Experimental Value (after 7 Days) | Predicted Value | |||||
| 7 Days | 10 Years | 200 Years | Finally | ||||
| 1 | 0.0538 | −0.0042 | −0.0010 | 0.0063 | 0.0264 | 0.0264 | 49.15 |
| 2 | 0.0230 | −0.0049 | −0.0008 | −0.0066 | 0.0007 | 0.0055 | 23.78 |
| 3 | 0.0365 | −0.0034 | −0.0011 | 0.0047 | −0.0329 | −0.0329 | −90.19 |
| 4 | 0.0292 | −0.0221 | −0.0011 | −0.0638 | −0.0625 | −0.0625 | −213.79 |
| 5 | 0.0498 | −0.0239 | −0.0012 | −0.0106 | −0.0355 | −0.0355 | −71.22 |
| 6 | 0.0385 | −0.0132 | −0.0015 | −0.0047 | −0.0436 | −0.0491 | −127.39 |
| 7 | 0.0500 | −0.0136 | −0.0006 | 0.0008 | 0.0217 | 0.0368 | 73.55 |
| 8 | 0.0446 | 0.0122 | −0.0006 | −0.0110 | −0.0433 | −0.0433 | −97.04 |
| 9 | 0.0421 | −0.0276 | −0.0015 | −0.0091 | −0.0412 | −0.0412 | −97.86 |
| 10 | 0.0349 | −0.0015 | −0.0013 | −0.0034 | −0.0011 | −0.0011 | −3.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, M.; Tu, Z.; Sun, X.; Yan, Z.; Feng, S.; Zhang, D.; Li, F.; Zhang, L. Experimental Study on CO2 Geochemical Reaction Characteristics in Marine Weakly Consolidated Sandstone Saline Aquifers. Processes 2023, 11, 3345. https://doi.org/10.3390/pr11123345
Xie M, Tu Z, Sun X, Yan Z, Feng S, Zhang D, Li F, Zhang L. Experimental Study on CO2 Geochemical Reaction Characteristics in Marine Weakly Consolidated Sandstone Saline Aquifers. Processes. 2023; 11(12):3345. https://doi.org/10.3390/pr11123345
Chicago/Turabian StyleXie, Mingying, Zhiyong Tu, Xiaona Sun, Zhenghe Yan, Shasha Feng, Deng Zhang, Fuyang Li, and Liang Zhang. 2023. "Experimental Study on CO2 Geochemical Reaction Characteristics in Marine Weakly Consolidated Sandstone Saline Aquifers" Processes 11, no. 12: 3345. https://doi.org/10.3390/pr11123345