Detection Limits of Antibiotics in Wastewater by Real-Time UV–VIS Spectrometry at Different Optical Path Length
Abstract
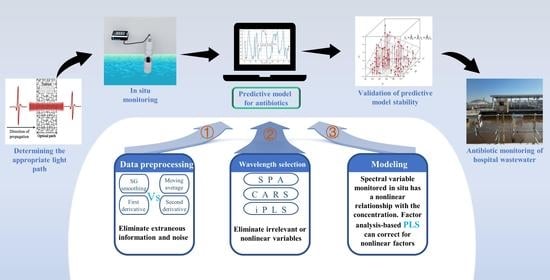
:1. Introduction
2. Materials and Methods
2.1. Subsection
2.2. Wastewater Samples
2.3. Modeling
2.3.1. Data Preprocessing
2.3.2. Wavelength Selection
3. Results and Discussion
3.1. LODs of Antibiotics at Different Optical Path Lengths
3.2. Detection of Antibiotics in Wastewater Using UV–Vis Spectroscopy
3.3. Model Establishment of Mixed Samples
3.3.1. Data Preprocessing
3.3.2. Wavelength Selection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Phoon, B.L.; Ong, C.C.; Mohamed Saheed, M.S.; Show, P.L.; Chang, J.S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and Emerging Technologies for Removal of Antibiotics from Wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, L.; Pitarch, E.; Fonseca, E.; Ibáñez, M.; Botero, A.M.; Claros, J.; Pastor, L.; Hernández, F. Investigation of Pharmaceuticals in a Conventional Wastewater Treatment Plant: Removal Efficiency, Seasonal Variation and Impact of a Nearby Hospital. J. Environ. Chem. Eng. 2021, 9, 105548. [Google Scholar] [CrossRef]
- Maribas, A.; da Silva, M.D.C.L.; Laurent, N.; Loison, B.; Battaglia, P.; Pons, M.N. Monitoring of Rain Events with a Submersible UV/VIS Spectrophotometer. Water Sci. Technol. 2008, 57, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Waltham, B.; Örmeci, B. Fluorescence Intensity, Conductivity, and UV–Vis Absorbance as Surrogate Parameters for Real-Time Monitoring of Anaerobic Digestion of Wastewater Sludge. J. Water Process Eng. 2020, 37, 101395. [Google Scholar] [CrossRef]
- Villez, K.; Vanrolleghem, P.A.; Corominas, L. Optimal Flow Sensor Placement on Wastewater Treatment Plants. Water Res. 2016, 101, 75–83. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Ye, R.; Duan, Q. Advances on Water Quality Detection by Uv-Vis Spectroscopy. Appl. Sci. 2020, 10, 6874. [Google Scholar] [CrossRef]
- Chen, B.; Wu, H.; Li, S.F.Y. Development of Variable Pathlength UV-Vis Spectroscopy Combined with Partial-Least-Squares Regression for Wastewater Chemical Oxygen Demand (COD) Monitoring. Talanta 2014, 120, 325–330. [Google Scholar] [CrossRef]
- Abitan, H.; Bohr, H.; Buchhave, P. Correction to the Beer-Lambert-Bouguer Law for Optical Absorption. Appl. Opt. 2008, 47, 5354–5357. [Google Scholar] [CrossRef]
- Yan, C.; Yang, Y.; Zhou, J.; Liu, M.; Nie, M.; Shi, H.; Gu, L. Antibiotics in the Surface Water of the Yangtze Estuary: Occurrence, Distribution and Risk Assessment. Environ. Pollut. 2013, 175, 22–29. [Google Scholar] [CrossRef]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A Review on Environmental Monitoring of Water Organic Pollutants Identified by EU Guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar]
- Gulyas, H.; Reich, M. Organic Constituents of Oil Reclaiming Wastewater. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2000, 35, 435–464. [Google Scholar] [CrossRef]
- Khan, M.F.S.; Akbar, M.; Wu, J.; Xu, Z. A Review on Fluorescence Spectroscopic Analysis of Water and Wastewater. Methods Appl. Fluoresc. 2022, 10, 012001. [Google Scholar] [CrossRef] [PubMed]
- Zamora, D.; Torres, A. Method for Outlier Detection: A Tool to Assess the Consistency between Laboratory Data and Ultraviolet- Visible Absorbance Spectra in Wastewater Samples. Water Sci. Technol. 2014, 69, 2305–2314. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin Biochem Rev. 2008, 29, 49–52. [Google Scholar]
- Environmental Quality Standards for Surface Water. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/shjzlbz/200206/t20020601_66497.htm (accessed on 12 December 2003).
- Filella, M.; Quentel, F.; Pernet-Coudrier, B.; Varrault, G. Application of a Refractory Organic Matter Quantification Method to Wastewater Effluents. Int. J. Environ. Anal. Chem. 2009, 89, 799–807. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Shi, Y.; Deng, C.; Hu, C.; Dong, Y.; Zhang, X.; Wang, T. Optical Path Optimal Length for Ultraviolet Ammonia Detection with a Compact and Flexible Gas-Absorption Cell. IEEE Sens. J. 2021, 21, 17889–17897. [Google Scholar] [CrossRef]
- Chen, X.; Yin, G.; Zhao, N.; Gan, T.; Yang, R.; Xia, M.; Feng, C.; Chen, Y.; Huang, Y. Simultaneous Determination of Nitrate, Chemical Oxygen Demand and Turbidity in Water Based on UV–Vis Absorption Spectrometry Combined with Interval Analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 244, 118827. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, M.; Yamashita, Y.; Funatsu, K. Genetic Algorithm-Based Wavelength Selection Method for Spectral Calibration. J. Chemom. 2011, 25, 10–19. [Google Scholar] [CrossRef]
- Şahin, S.; Sariburun, E.; Demir, C. Net Analyte Signal-Based Simultaneous Determination of Dyes in Environmental Samples Using Moving Window Partial Least Squares Regression with UV-Vis Spectroscopy. Anal. Methods 2009, 1, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Technical Guidelines for Hospital Wastewater Treatment of China. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/other/hjbhgc/200312/t20031210_88352.shtml (accessed on 1 June 2002).
- Guo, H.; Li, X.; Ren, Q.; Liu, S.; Wu, Y.; Jia, L. Characteristic Analysis of Influent Water Quality of Typical Urban Wastewater Treatment Plants in China. Water Wastewater Eng. 2018, 54, 12–15. [Google Scholar] [CrossRef]
- Rinnan, Å.; van den Berg, F.; Engelsen, S.B. Review of the Most Common Pre-Processing Techniques for near-Infrared Spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Rinnan, Å. Pre-Processing in Vibrational Spectroscopy-When, Why and How. Anal. Methods 2014, 6, 7124–7129. [Google Scholar] [CrossRef]
- Tiecher, T.; Moura-Bueno, J.M.; Caner, L.; Minella, J.P.G.; Evrard, O.; Ramon, R.; Naibo, G.; Barros, C.A.P.; Silva, Y.J.A.B.; Amorim, F.F.; et al. Improving the Quantification of Sediment Source Contributions Using Different Mathematical Models and Spectral Preprocessing Techniques for Individual or Combined Spectra of Ultraviolet–Visible, near- and Middle-Infrared Spectroscopy. Geoderma 2021, 384, 114815. [Google Scholar] [CrossRef]
- Irving, H.M.N.H.; Freiser, H.; West, T.S. Compendium of Analytical Nomenclature; Blackwell Scientific Publications: Boston, MA, USA, 1978; pp. 174–175. [Google Scholar]
- Garrido Frenich, A.; Jouan-Rimbaud, D.; Massart, D.L.; Kuttatharmmakul, S.; Martínez Galera, M.; Martínez Vidal, J.L. Wavelength Selection Method for Multicomponent Spectrophotometric Determinations Using Partial Least Squares. Analyst 1995, 120, 2787–2792. [Google Scholar] [CrossRef]
- Nørgaard, L.; Saudland, A.; Wagner, J.; Nielsen, J.P.; Munck, L.; Engelsen, S.B. Interval Partial Least-Squares Regression (IPLS): A Comparative Chemometric Study with an Example from near-Infrared Spectroscopy. Appl. Spectrosc. 2000, 54, 413–419. [Google Scholar] [CrossRef]
- Soares, S.F.C.; Gomes, A.A.; Araujo, M.C.U.; Filho, A.R.G.; Galvão, R.K.H. The Successive Projections Algorithm. TrAC Trends Anal. Chem. 2013, 42, 84–98. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Xu, Q.; Cao, D. Key Wavelengths Screening Using Competitive Adaptive Reweighted Sampling Method for Multivariate Calibration. Anal. Chim. Acta 2009, 648, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.X.; Fang, Z.; Min, R.; Bai, X.; Zhang, Y.; Xu, D.; Fang, P.Q. Is Nationwide Special Campaign on Antibiotic Stewardship Program Effective on Ameliorating Irrational Antibiotic Use in China? Study on the Antibiotic Use of Specialized Hospitals in China in 2011–2012. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 456–463. [Google Scholar] [CrossRef]
- Li, D. Trend Analysis of Antibiotic Use in China’s General Hospitals during 2012–2016. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2019. [Google Scholar] [CrossRef]
- Tang, X.; Zuo, J.; Yu, X.; Yang, B.; Wang, K. Residue Analysis Method and Environmental Risk Assessment of Cephalosporin Antibiotics in Pharmaceutical. China Environ. Sci. 2014, 34, 2273–2278. [Google Scholar]
- Insausti, M.; Gomes, A.A.; Cruz, F.v.; Pistonesi, M.F.; Araujo, M.C.U.; Galvão, R.K.H.; Pereira, C.F.; Band, B.S.F. Screening Analysis of Biodiesel Feedstock Using UV-Vis, NIR and Synchronous Fluorescence Spectrometries and the Successive Projections Algorithm. Talanta 2012, 97, 579–583. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Fan, W.; Lv, H.; Liang, Y.; Shan, Y.; Li, G.; Yang, Z.; Yu, L. Simultaneous Determination of Traces Amounts of Cadmium, Zinc, and Cobalt Based on UV-Vis Spectrometry Combined with Wavelength Selection and Partial Least Squares Regression. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 123, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yin, H.; Wang, K.; Dong, H.; Hou, D. Adaptive Detection Method for Organic Contamination Events in Water Distribution Systems Using the UV-Vis Spectrum Based on Semi-Supervised Learning. Water 2018, 10, 1566. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Liu, J.; Bian, X.; Zhang, Q.; Pan, J.; Wan, D. UV–Vis Spectroscopic Detection Coupled with Chemometrics for the Measurement of Mixed Organic Acids in Water Samples Enriched by Radial Electric Focusing Solid Phase Extraction. Metrol. Meas. Syst. 2018, 25, 317–329. [Google Scholar] [CrossRef]
- Hoskuldsson, A. A combined theory for PCA and PLS. J. Chemom. 1995, 9, 91–123. [Google Scholar] [CrossRef]
- Li, X.; Mei, Q.; Yan, X.; Dong, B.; Dai, X.; Yu, L.; Wang, Y.; Ding, G.; Yu, F.; Zhou, J. Molecular Characteristics of the Refractory Organic Matter in the Anaerobic and Aerobic Digestates of Sewage Sludge. RSC Adv. 2018, 8, 33138–33148. [Google Scholar] [CrossRef] [PubMed]





| Sensor 1 | Sensor 2 | |
|---|---|---|
| Spectrometer Probe |  |  |
| Lamp type | Xenon lamp | Xenon lamp |
| Optical path | 0.5 mm | 1–10 cm |
| Control platforms | Moni::tool V3.1.4 | LabVIEW 2018 |
| Resolution | 2.5 nm | 0.5 nm |
| Scan interval | 70 s | 30 s |
| Scan range | 200–737.5 nm | 186.5–665.5 nm |
| COD (mg/L) | BOD5 (mg/L) | SS (mg/L) | NH3–N (mg/L) | |
|---|---|---|---|---|
| Hospital wastewater 1 | 150–300 | 80–150 | 40–120 | 10–50 |
| WWTP influent water 2 | 219.97 | 81.64 | 148.54 | 22.83 |
| Wastewater using in this study | 211 | 103 | 143 | 20.42 |
| Mean Value of Blank Sample (1/m) | Standard Deviation of Blank Sample (1/m) | Sensitivity | Limit of Detection (mg/L) | |
|---|---|---|---|---|
| 280 nm (Tetracycline) 1 | 1.26604 | 0.99657 | 3.198 | 0.9349 |
| 290 nm (Ofloxacin) 1 | 1.07527 | 1.01339 | 6.542 | 0.4647 |
| 280 nm (Chloramphenicol) 1 | 1.26604 | 0.99657 | 2.640 | 1.1325 |
| 273 nm (Tetracycline) 2 | −0.00429 | 0.01089 | 10.560 | 0.0031 |
| 286 nm (Ofloxacin) 2 | −0.00259 | 0.01052 | 6.990 | 0.0045 |
| 271 nm (Chloramphenicol) 2 | −0.00159 | 0.00986 | 0.310 | 0.0954 |
| Optical Path Length (cm) | Limit of Detection (μg/L) | ||
|---|---|---|---|
| Tetracycline | Ofloxacin | Chloramphenicol | |
| 10 | 3.1 | 4.5 | 95.4 |
| 5 | 18.4 | 25.3 | 137.5 |
| 3 | 43.7 | 58.9 | 173.8 |
| 1 | 75.4 | 95.1 | 241.2 |
| 0.05 | 934.9 | 464.7 | 1132.5 |
| Pretreatment Method | Ofloxacin | Tetracycline | Chloramphenicol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LVs | RMSEP | R2 | LVs | RMSEP | R² | LVs | RMSEP | R² | |
| No pretreatment | 4 | 3.16105 | 0.98799 | 3 | 1.51881 | 0.99699 | 3 | 3.33167 | 0.99015 |
| SG smoothing | 4 | 3.46094 | 0.98571 | 3 | 1.55338 | 0.99594 | 5 | 3.3529 | 0.99011 |
| Moving average | 3 | 3.02822 | 0.98959 | 4 | 1.56447 | 0.99673 | 3 | 0.80787 | 0.99941 |
| First derivative | 4 | 3.28151 | 0.98764 | 3 | 0.84661 | 0.99905 | 5 | 0.72231 | 0.99957 |
| Second derivative | 4 | 2.28809 | 0.99311 | 5 | 1.84422 | 0.99519 | 5 | 4.46114 | 0.98245 |
| Wavelength Selection Method | Ofloxacin | Tetracycline | Chloramphenicol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ws 1 | RMSEP | R² | Ws 1 | RMSEP | R² | Ws 1 | RMSEP | R² | |
| SPA | 5 | 3.30572 | 0.98800 | 5 | 2.45338 | 0.99094 | 3 | 8.51275 | 0.93337 |
| CARS | 16 | 2.49126 | 0.99281 | 6 | 0.84635 | 0.99873 | 13 | 3.74908 | 0.98767 |
| iPLS | 9 | 1.88800 | 0.99528 | 9 | 1.75843 | 0.99600 | 28 | 4.13771 | 0.98305 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Wang, X.; Yang, M.; Zhu, M.; Chen, W.; Li, Q.; Sun, D.; Bi, X.; Maletskyi, Z.; Ratnaweera, H. Detection Limits of Antibiotics in Wastewater by Real-Time UV–VIS Spectrometry at Different Optical Path Length. Processes 2022, 10, 2614. https://doi.org/10.3390/pr10122614
Li F, Wang X, Yang M, Zhu M, Chen W, Li Q, Sun D, Bi X, Maletskyi Z, Ratnaweera H. Detection Limits of Antibiotics in Wastewater by Real-Time UV–VIS Spectrometry at Different Optical Path Length. Processes. 2022; 10(12):2614. https://doi.org/10.3390/pr10122614
Chicago/Turabian StyleLi, Feng, Xiaodong Wang, Manzi Yang, Ming Zhu, Wei Chen, Qiran Li, Delin Sun, Xuejun Bi, Zakhar Maletskyi, and Harsha Ratnaweera. 2022. "Detection Limits of Antibiotics in Wastewater by Real-Time UV–VIS Spectrometry at Different Optical Path Length" Processes 10, no. 12: 2614. https://doi.org/10.3390/pr10122614