The Influence of Solvent and Extraction Time on Yield and Chemical Selectivity of Cuticular Waxes from Quercus suber Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Cuticular Wax Extraction
2.3. Cuticular Wax Composition
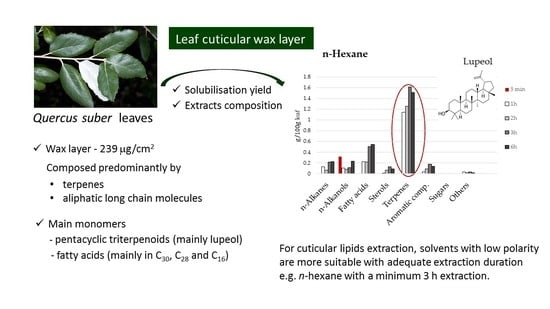
3. Results and Discussion
3.1. Extraction Yields
3.2. Effect of Solvent on Lipid Extraction
3.3. Compositional Analysis of Extracts by Chemical Family
3.4. Chemical Composition of Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollard, M.; Beisson, F.; Li, Y.; Ohlrogge, J.B. Building lipid barriers: Biosynthesis of cutin and suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Buschhaus, C.; Jetter, R. Composition differences between epicuticular and intracuticular wax substructures: How do plants seal their epidermal surfaces? J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffree, C.E. The fine structure of the plant cuticle. In Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; Annual Plant Review; Blackwell: Oxford, UK, 2006; Volume 23, pp. 11–125. [Google Scholar]
- Schreiber, L.; Schönherr, J. Water and Solute Permeability of Plant Cuticles: Measurement and Data Analysis; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Jetter, R.; Riederer, M. Localization of the transpiration barrier in the epi- and intracuticular waxes of eight plant species: Water transport resistances are associated with fatty acyl rather than alicyclic components. Plant Physiol. 2016, 170, 921–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisler-Diehl, V.; Müller, Y.; Schreiber, L. Epicuticular wax on leaf cuticles does not establish the transpiration barrier, which is essentially formed by intracuticular wax. J. Plant Physiol. 2018, 227, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Bourgault, R.; Matschi, S.; Vasquez, M.; Qiao, P.; Sonntag, A.; Charlebois, C.; Mohammadi, M.; Scanlon, M.J.; Smith, L.G.; Molina, I. Constructing functional cuticles: Analysis of relationships between cuticle lipid composition, ultrastructure and water barrier function in developing adult maize leaves. Ann. Bot. 2020, 125, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, H. Cork: Biology, Production and Uses; Elsevier Science: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Martins, C.M.C.; Mesquita, S.M.M.; Vaz, W.L.C. Cuticular waxes of the holm (Quercus ilex L. subsp. ballota (Desf.) Samp.) and cork (Q. suber L.) oaks. Phytochem. Anal. 1999, 10, 1–5. [Google Scholar] [CrossRef]
- Simões, R.; Rodrigues, A.; Ferreira-Dias, S.; Miranda, I.; Perreira, H. Chemical composition of cuticular waxes and pigments and morphology of leaves of Quercus suber trees of different provenance. Plants 2020, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.A.; Heras, B.; García, M.D.; Sáenz, M.T.; Villar, A. New insights into the mechanism of action of the anti-inflammatory triterpene lupeol. J. Pharm. Pharmacol. 2001, 53, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Lu, H.; Lu, J.; Chen, G.; Yokoyama, T.; Sagara, Y.; Manabe, M.; Kodama, H. Effect of three triterpenoids, lupeol, betulin, and betulinic acid on the stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils. Clin. Chim. Acta 2002, 325, 91–96. [Google Scholar] [CrossRef]
- Prasad, S.; Kumar, Y.V.; Srivastava, S.; Shukla, Y. Protective effects of lupeol against benzo[a]pyrene induced clastogenicity in mouse bone marrow cells. Mol. Nutr. Food Res. 2008, 52, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Siddique, H.R.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Khan, A.; Lee, H.J.; Rehman, I.U.; Khan, I.; Alam, S.I.; Kim, M.O. Lupeol, a plant-derived triterpenoid, protects mice brains against Aβ-induced oxidative stress and neurodegeneration. Biomedicines 2020, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Jetter, R.; Schäffer, S.; Riederer, M. Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: Evidence from Prunus laurocerasus L. Plant Cell Environ. 2000, 23, 619–628. [Google Scholar] [CrossRef]
- Sharma, P.; Kothari, S.L.; Rathore, M.; Gour, V. Properties, variations, roles, and potential applications of cuticular wax: A review. Turk. J. Bot. 2018, 42, 135–149. [Google Scholar] [CrossRef]
- Yeats, T.H.; Rose, J.K. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loneman, D.M.; Peddicord, L.; Al-Rashid, A.; Nikolau, B.J.; Lauter, N.; Yandeau-Nelson, M.D. A robust and efficient method for the extraction of plant extra cellular surface lipids as applied to the analysis of silks and seedling leaves of maize. PLoS ONE 2017, 12, e0180850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, V.; Guzmán-Delgado, P.; Graça, J.; Santos, S.; Gil, L. Cuticle structure in relation to chemical composition: Re-assessing the prevailing m7odel. Front. Plant Sci. 2016, 7, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abouzeid, S.; Beutling, U.; Selmar, D. Stress-induced modification of indole alkaloids: Phytomodificines as a new category of specialized metabolites. Phytochemistry 2019, 159, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Fenili, D.; Brown, M.; Rappaport, R.; McLaurin, J. Properties of scyllo-inositol as a therapeutic treatment of AD-like pathology. J. Mol. Med. 2007, 85, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, W.S.; Lim, S.; Kim, Y.K.; Jung, H.-Y.; Das, S.; Lee, J.; Luo, W.; Kim, K.-T.; Chung, S.-K. A guanidine-appended scyllo-inositol derivative AAD-66 enhances brain delivery and ameliorates Alzheimer’s phenotypes. Sci. Rep. 2017, 7, 14125. [Google Scholar] [CrossRef] [PubMed]


| 5 min | 1 h | 2 h | 3 h | 6 h | |
|---|---|---|---|---|---|
| n-Hexane | |||||
| g/100 g leaves | 0.33 ± 0.04 | 1.92 ± 0.72 | 1.98 ± 0.25 | 3.11 ± 0.11 | 3.39 ± 0.19 |
| μg/cm2 | 28.13 ± 3.09 | 165.67 ± 62.33 | 170.58 ± 21.52 | 286.02 ± 9.27 | 287.15 ± 23.18 |
| Dichloromethane | |||||
| g/100 g leaves | 0.51 ± 0.03 | 1.17 ± 0.09 | 1.23 ± 0.13 | 1.79 ± 0.33 | 2.77 ± 0.17 |
| μg/cm2 | 43.83 ± 2.88 | 101.36 ± 7.66 | 108.30 ± 11.17 | 154.10 ± 28.52 | 239.00 ± 14.25 |
| Acetone | |||||
| g/100 g leaves | 0.35 ± 0.07 | 1.27 ± 0.25 | 2.70 ± 0.15 | 3.16 ± 0.02 | 3.57 ± 0.49 |
| μg/cm2 | 30.29 ± 5.84 | 109.42 ± 21.81 | 232.86 ± 12.81 | 272.24 ± 2.12 | 308.33 ± 42.32 |
| Proportion of Total Compounds, % | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n-Hexane | Dichloromethane | Acetone | |||||||||||||
| Family | 5 min | 1 h | 2 h | 3 h | 6 h | 5 min | 1 h | 2 h | 3 h | 6 h | 5 min | 1 h | 2 h | 3 h | 6 h |
| Alkanes | 0.3 | 6.9 | 3.5 | 6.9 | 6.6 | 0.4 | 3.4 | 3.1 | 2.2 | 3.6 | - | 0.5 | 1.8 | 0.4 | 0.2 |
| Alkanols | 98.4 | 5.5 | 4.0 | 3.7 | 7.0 | 95.5 | 5.1 | 7.3 | 5.5 | 7.3 | 2.1 | 3.8 | 3.7 | 4.2 | 2.0 |
| Fatty acids | 0.2 | 11.8 | 11.0 | 16.3 | 16.1 | 0.9 | 12.6 | 25.0 | 25.3 | 20.0 | 39.6 | 5.4 | 11.6 | 11.7 | 9.0 |
| Glycerides | - | 0.1 | 0.2 | 0.5 | 0.3 | - | 1.0 | 2.5 | 0.9 | 0.2 | - | 1.1 | 1.9 | 1.8 | 1.2 |
| Sterols | - | 0.01 | 3.2 | 4.2 | 2.7 | - | 2.0 | 3.0 | 2.7 | 3.4 | - | 1.1 | 1.5 | 1.6 | 1.2 |
| Terpenes | 0.1 | 59.7 | 63.4 | 52.0 | 44.6 | 2.0 | 59.9 | 42.7 | 48.4 | 44.8 | - | 19.5 | 20.3 | 20.5 | 22.2 |
| Aromatics | - | 1.7 | 4.5 | 5.6 | 4.1 | - | 4.7 | 5.6 | 5.7 | 4.5 | 13.5 | 2.9 | 3.2 | 2.6 | 2.7 |
| Sugars | - | 0.0 | 0.0 | 0.0 | 0.0 | - | 0.1 | 0.5 | 1.4 | 0.6 | 39.7 | 56.8 | 50.0 | 41.3 | 46.8 |
| Others | - | 1.6 | 0.9 | 0.6 | 0.3 | - | 1.1 | 0.5 | 1.4 | 1.4 | 5.0 | 6.2 | 1.7 | 9.9 | 8.4 |
| Total Identified | 99.0 | 87.3 | 90.7 | 89.8 | 81.7 | 99.5 | 90.0 | 90.7 | 93.7 | 85.5 | 99.8 | 93.4 | 92.0 | 89.7 | 91.6 |
| Extraction Time | |||||
|---|---|---|---|---|---|
| Wax Constituent (% Total Peak Area) | 5 min | 1 h | 2 h | 3 h | 6 h |
| Alkanes | |||||
| Heptacosane (C27) | - | - | - | 1.12 | 1.02 |
| Triacontane (C30) | - | 5.10 | 2.47 | 4.89 | 4.66 |
| Alkanols | |||||
| Hexadecan-1-ol (C16OH) | - | 1.15 | - | - | - |
| Docosan-1-ol (C22OH) | 1.24 | - | - | - | - |
| Tretracosan-1-ol (C24OH) | 96.64 | 2.91 | 3.11 | 3.05 | 4.34 |
| Fatty acids | |||||
| Saturated | |||||
| Hexadecanoic acid (C16:0) | - | 7.57 | 5.35 | 5.57 | 5.32 |
| Hexacosanoic acid (C26:0) | - | - | - | 1.00 | 1.05 |
| Octacosanoic acid (C28:0) | - | - | - | 2.61 | 3.39 |
| Triacontanoic acid (C30:0) | - | - | - | 1.08 | 1.37 |
| Unsaturated | |||||
| 9,12-Octadecadienoic acid (C18:2) | - | - | - | 1.12 | 0.81 |
| 9,12,15-Octadecatrienoic acid (C18:3) | - | - | 2.28 | 3.43 | 2.11 |
| Sterols | |||||
| β-Systosterol | - | - | 2.82 | 3.50 | 2.50 |
| Terpenes | |||||
| pentacyclic triterpenes | |||||
| β-Amyrin | - | 3.02 | 2.83 | 3.11 | 2.47 |
| Germanicol | - | 11.19 | 17.31 | 7.49 | 6.00 |
| Lupeol | - | 43.93 | 40.43 | 37.76 | 33.04 |
| Betulin | - | - | - | 1.12 | 0.97 |
| Aromatics | |||||
| Hexadecyl-(E)-p-coumarate | - | - | 4.21 | 5.32 | 3.66 |
| Extraction Time | |||||
|---|---|---|---|---|---|
| Wax Constituent (% Total Peak Area) | 5 min | 1 h | 2 h | 3 h | 6 h |
| Alkanes | |||||
| Nonacosane (C29) | - | 2.53 | 2.18 | 1.47 | 2.56 |
| Alkanols | |||||
| Hexadecan-1-ol (C16OH) | 0.90 | - | - | - | - |
| Docosan-1-ol (C22OH) | 2.38 | 0.77 | 1.12 | 1.10 | 1.85 |
| Tretracosan-1-ol (C24OH) | 92.12 | 3.47 | 4.59 | 3.31 | 4.62 |
| Fatty acids | |||||
| Saturated | |||||
| Hexadecanoic acid (C16:0) | - | 7.60 | 9.84 | 9.71 | 9.68 |
| Octacosanoic acid (C28:0) | - | 1.60 | 2.31 | 1.14 | 2.49 |
| Unsaturated | |||||
| 9,12-Octadecadienoic acid (C18:2) | - | 0.14 | 1.73 | 2.38 | 0.75 |
| 9,12,15-Octadecatrienoic acid (C18:3) | - | 0.39 | 6.43 | 8.04 | 2.69 |
| Glycerides | |||||
| Glycerol | - | 0.95 | 2.14 | 0.51 | - |
| Sterols | |||||
| β-Systosterol | - | 1.59 | 2.55 | 2.33 | 3.24 |
| Terpenes | |||||
| Pentacyclic triterpenes | |||||
| β-Amyrin | - | 2.75 | 2.29 | 2.56 | 2.11 |
| Germanicol | - | 9.15 | 5.46 | 6.46 | 4.77 |
| Lupeol | 1.61 | 45.44 | 31.56 | 35.28 | 35.37 |
| Aromatics | |||||
| Hexadecyl-(E)-p-coumarate | - | 4.27 | 5.31 | 5.19 | 4.09 |
| Sugars | |||||
| Fructofuranose | - | 0.09 | 0.54 | 1.43 | 0.18 |
| Extraction Time | |||||
|---|---|---|---|---|---|
| Wax Constituent (% Total Peak Area) | 5 min | 1 h | 2 h | 3 h | 6 h |
| Alkanols | |||||
| 1,4-Butanediol | 1.85 | - | - | - | - |
| Tretracosan-1-ol (C24OH) | - | 3.22 | 3.21 | 2.74 | 1.28 |
| Fatty acids | |||||
| Saturated | |||||
| Hexadecanoic acid (C16:0) | - | 2.51 | 5.54 | 5.92 | 4.35 |
| Unsaturated | |||||
| 9,12-Octadecadienoic acid (C18:2) | - | 0.03 | 0.66 | 1.23 | 0.48 |
| 9,12,15-Octadecatrienoic acid (C18:3) | - | 0.07 | 0.61 | 0.07 | 1.26 |
| Diacids | |||||
| Butanedioic acid (C4:0) | - | 0.95 | 0.99 | 2.45 | 1.69 |
| Glycerides | |||||
| Glycerol | - | 1.04 | 1.72 | 1.36 | 0.95 |
| Sterols | |||||
| β-Systosterol | - | 0.32 | 0.99 | 1.43 | 1.15 |
| Terpenes | |||||
| pentacyclic triterpenes | |||||
| β-Amyrin | - | 0.72 | - | 1.18 | 1.19 |
| Germanicol | - | 2.89 | 2.05 | 2.71 | 2.86 |
| Lupeol | - | 14.53 | 16.25 | 14.71 | 16.15 |
| Aromatics | |||||
| Catechine | 13.18 | - | - | - | - |
| Hexadecyl-(E)-p-coumarate | 1.67 | 1.62 | 2.35 | 2.42 | |
| Sugars | |||||
| Myo Inositol | 19.98 | - | 15.34 | - | −0.11 |
| Scyllo Inositol | - | 15.44 | 14.27 | 12.39 | 14.88 |
| Deoxinositol | - | 6.28 | 1.81 | 5.24 | 6.3 |
| D-Fructose | - | 15.47 | 11.27 | 10.68 | 8.61 |
| (α/β) D-Glucopyranose (isomer) | - | 17.11 | 6.28 | 11.18 | 15.01 |
| Sucrose | 18.62 | 0.48 | 0.21 | 0.47 | 0.4 |
| Others compounds | |||||
| Quininic acid | 38.18 | 4.6 | 0.94 | 7.2 | 7.15 |
| Shikimic acid, 4TMS derivative | 5.03 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simões, R.; Miranda, I.; Pereira, H. The Influence of Solvent and Extraction Time on Yield and Chemical Selectivity of Cuticular Waxes from Quercus suber Leaves. Processes 2022, 10, 2270. https://doi.org/10.3390/pr10112270
Simões R, Miranda I, Pereira H. The Influence of Solvent and Extraction Time on Yield and Chemical Selectivity of Cuticular Waxes from Quercus suber Leaves. Processes. 2022; 10(11):2270. https://doi.org/10.3390/pr10112270
Chicago/Turabian StyleSimões, Rita, Isabel Miranda, and Helena Pereira. 2022. "The Influence of Solvent and Extraction Time on Yield and Chemical Selectivity of Cuticular Waxes from Quercus suber Leaves" Processes 10, no. 11: 2270. https://doi.org/10.3390/pr10112270