Quality Changes of Low-Density Polyethylene (LDPE) Recyclates from the Pretreatment Process with a Cationic Surfactant and a Nonionic Surfactant as Cleaning Agents Upstream of Extrusion
Abstract
:1. Introduction
2. Backgrounds
2.1. Cleaning with Surfactants as Pretreatment Process
2.2. Printing Ink Compositions
3. Materials and Methods
3.1. Materials
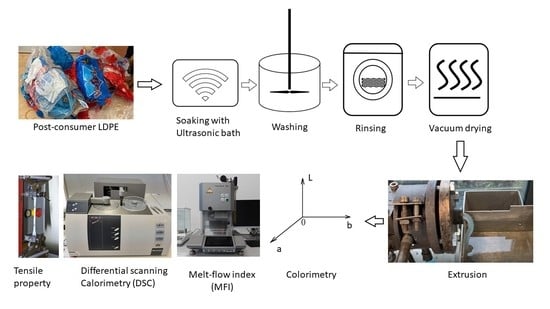
3.2. Cleaning Experiment Setup
3.2.1. Small-Scale Experiment
3.2.2. Scale-Up
3.3. Cleaning Performance Evaluation
3.3.1. CIELAB (International Commission on Illumination) Method
- DE*: de-inking rate;
- : DE* value of reference sample (virgin polymer)
- L: the value of lightness;
- a: green-red opponent colors, a negative value for greenish and a positive value for reddish;
- b: blue-yellow opponent colors, a negative value for blueish and a positive value for yellowish.
3.3.2. Flake Sorting Method
- DE*: de-inking rate
- mct: mass of completely transparent flakes (g);
- mpt: mass of partly transparent flakes (g);
- msample: mass of the whole selected flakes (g).
3.4. Quality Analysis of Recyclate
- χc: Crystallinity in %;
- ∆H: Enthalpy of fusion (J/g);
- ∆Hc,100%: Enthalpy of fusion assuming 100% crystallinity, here 293 J/g [66].
- A tensile strength testing machine (Zwick & Roell Z2.5) was applied to measure the elongation modulus, the tensile strength, and strain at the break of the recyclates according to ISO 527 [67]. Each sample was measured with five replications.
3.5. Evaluation of the Substitutability
- RQmech: recycling quality score of the mechanical property;
- RQE: recycling quality score of the E-modulus;
- : recycling quality score of the yield strength;
- : recycling quality score of the strain at break.
4. Results
4.1. De-Inking Effect of Small-Scale Experiment
4.2. De-Inking Effect of Large-Scale Experiment
4.3. Material Test
4.3.1. Melt Flow Index (MFI)
4.3.2. DSC Analysis
4.3.3. Tensile Strength
5. Discussion
5.1. Cleaning Effectiveness of Different Conditions
5.2. De-Inking Effect and Recyclate Quality
5.3. Substitutability Evaluation
5.4. Limitations of This Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe. An analysis of European plastics production, demand and waste data. In Plastics—The Facts 2020; Plastics Europe: Brussels, Belgium, 2020. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Lavender, K. Law, Production, use and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceresana. Polyethylene LDPE Market Report; Ceresana Market Research: Konstanz, Germany, 2020; Available online: https://www.ceresana.com/en/market-studies/plastics/polyethylene-ldpe/ceresana-market-study-polyethylene-ldpe.html (accessed on 21 October 2021).
- Antonopoulos, I.; Faraca, G.; Tonini, D. Recycling of post-consumer plastic packaging waste in the EU: Recovery rates, material flows, and barriers. Waste Manag. 2021, 126, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2017, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Plastic Recycler Europe. Flexible Films Market in Europe: State of Play. Production, Collection and Recycling Data; Plastic Recycler Europe: Brussels, Belgium, 2020. [Google Scholar]
- Feil, A.; Pretz, T.; Jansen, M.; Thoden van Velzen, E.U. Separate collection of plastic waste, better than technical sorting from municipal solid waste? Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2017, 35, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Ragossnig, A.M.; Schneider, D.R. What is the right level of recycling of plastic waste? Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2017, 35, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; Van Velzen, E.T.; Pretz, T. Handbook for Sorting of Plastic Packaging Waste Concentrates. Separation Efficiencies of Common Plastic Packaging Objects in Widely Used Separaion Machines at Existing Sorting Facilities with Mixed Post-Consumer Plastic Packaging Waste as Input; Wageningen UR Food & Biobased Research: Wageningen, The Netherlands, 2015. [Google Scholar]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Christiani, J.; Beckamp, S. Was Können die Mechanische Aufbereitung von Kunststoffen und das Werkstoffliche Recycling Leisten; Thomé-Kozmiensky Verlag GmbH: Neuruppin, Germany, 2020. [Google Scholar]
- Lange, J.-P. Managing Plastic Waste—Sorting, Recycling, Disposal, and Product Redesign. ACS Sustain. Chem. Eng. 2021, 9, 15722–15738. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities, Philosophical transactions of the Royal Society of London. Ser. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [Green Version]
- De Biasio, M.; Arnold, T.; Mcgunnigle, G.; Leitner, R.; Balthasar, D.; Rehrmann, V. Detecting and Discriminating PE and PP Polymers for Plastics Recycling Using NIR Imaging Spectroscopy; SPIE: Orlando, FL, USA, 2010. [Google Scholar]
- Hollstein, F.; Wohllebe, M.; Arnaiz, S. Identification and Sorting of Plastics Film Waste by NIR-Hyperspectral-Imaging. In Proceedings of the ICNIRS, Foz Do Iguassu, Brazil, 18–23 October 2015. [Google Scholar]
- Duan, Q.; Li, J. Classification of Common Household Plastic Wastes Combining Multiple Methods Based on Near-Infrared Spectroscopy. ACS ES T Eng. 2021, 1, 1065–1073. [Google Scholar] [CrossRef]
- Chen, X.; Kroell, N.; Feil, A.; Pretz, T. Determination of the composition of multilayer plastic packaging with NIR spectroscopy. Detritus 2020, 13, 62–66. [Google Scholar] [CrossRef]
- Mattley, Y.; Guenther, D. Sorting Polymers by Unique Spectral Features. Available online: https://www.oceaninsight.com/blog/spectroscopy-for-plastics-recycling/ (accessed on 28 August 2022).
- Trinamix: Distinguishing between HDPE and LDPE at the Blink of an Eye: New PE Applications for TrinamiX’s Mobile NIR Spectroscopy Solution. Available online: https://trinamixsensing.com/media/p175e_trinamix_hdpe_ldpe_distinguishing_pe_pp.pdf (accessed on 21 October 2021).
- Koinig, G.; Friedrich, K.; Rutrecht, B.; Oreski, G.; Barretta, C.; Vollprecht, D. Influence of reflective materials, emitter intensity and foil thickness on the variability of near-infrared spectra of 2D plastic packaging materials. Waste Manag. 2022, 144, 543–551. [Google Scholar] [CrossRef] [PubMed]
- COWI. Report: Study about Plastic Sorting and Recycling; COWI: Lyngby, Denmark, 2019. [Google Scholar]
- Dehoust, G.; Hermann, A.; Christiani, J.; Bartnik, S.; Beckamp, S.; Bünemann, A. Ermittlung der Praxis der Sortierung und Verwertung von Verpackungen im Sinne des § 21 VerpackG, Endbericht; Umweltbundesamt: Dessau-Roßlau, Germany, 2021. [Google Scholar]
- Alassali, A.; Picuno, C.; Chong, Z.K.; Guo, J.; Maletz, R.; Kuchta, K. Towards Higher Quality of Recycled Plastics: Limitations from the Material’s Perspective. Sustainability 2021, 13, 13266. [Google Scholar] [CrossRef]
- Nerin, C.; Alfaro, P.; Aznar, M.; Domeño, C. The challenge of identifying non-intentionally added substances from food packaging materials: A review. Anal. Chim. Acta 2013, 775, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Geueke, B. Fpf Dossier: Non-Intentionally Added Substances (Nias); Zenodo: Geneva, Switzerland, 2015. [Google Scholar] [CrossRef]
- Horodytska, O.; Cabanes, A.; Fullana, A. Non-intentionally added substances (NIAS) in recycled plastics. Chemosphere 2020, 251, 126373. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, A.-C.; Barenstedt, C.; Karlsson, S.; Lindberg, T. Degradation product pattern and morphology changes as means to differentiate abiotically and biotically aged degradable polyethylene. Polymer 1995, 36, 3075–3083. [Google Scholar] [CrossRef]
- Sugimoto, M.; Shimada, A.; Kudoh, H.; Tamura, K.; Seguchi, T. Product analysis for polyethylene degradation by radiation and thermal ageing. Radiat. Phys. Chem. 2013, 82, 69–73. [Google Scholar] [CrossRef]
- Rychlý, J.; Rychlá, L. Polyolefins: From Thermal and Oxidative Degradation to Ignition and Burning; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, e2000415. [Google Scholar] [CrossRef]
- Bradley, E.; Coulier, L. An Investigation into the Reaction and Breakdown Products from Starting Substances Used to Produce Food Contact Plastics. London, UK. 2007. Available online: https://www.foodpackagingforum.org/fpf-2016/wp-content/uploads/2014/06/Bradley-and-Coulier-2007.pdf (accessed on 28 August 2022).
- Pfaendner, R. Restabilization—30 years of research for quality improvement of recycled plastics review. Polym. Degrad. Stab. 2022, 203, 9–10. [Google Scholar] [CrossRef]
- EuPIA. Printing Inks and Plastic Recycling—Q & A; EuPIA: Brussels, Belgium, 2021. [Google Scholar]
- Hirschfeld, S.; Wünsch, O. Mass transfer during bubble-free polymer devolatilization: A systematic study of surface renewal and mixing effects. Heat Mass Transf. 2020, 56, 25–36. [Google Scholar] [CrossRef]
- Alshahrani, S.M.; Morott, J.T.; Alshetaili, A.S.; Tiwari, R.V.; Majumdar, S.; Repka, M.A. Influence of degassing on hot-melt extrusion process. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2015, 80, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Gall, M.; Freudenthaler, P.J.; Fischer, J.; Lang, R.W. Characterization of Composition and Structure–Property Relationships of Commercial Post-Consumer Polyethylene and Polypropylene Recyclates. Polymers 2021, 13, 1574. [Google Scholar] [CrossRef] [PubMed]
- Luijsterburg, B.J.; Jobse, P.S.; Spoelstra, A.B.; Goossens, J.G.P. Solid-state drawing of post-consumer isotactic poly(propylene): Effect of melt filtration and carbon black on structural and mechanical properties. Waste Manag. 2016, 54, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Roosen, M.; Harinck, L.; Ügdüler, S.; de Somer, T.; Hucks, A.-G.; Belé, T.G.A.; Buettner, A.; Ragaert, K.; van Geem, K.M.; Dumoulin, A.; et al. Deodorization of post-consumer plastic waste fractions: A comparison of different washing media. Sci. Total Environ. 2022, 812, 152467. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, A.; Strangl, M.; Ortner, E.; Fullana, A.; Buettner, A. Odorant composition of post-consumer LDPE bags originating from different collection systems. Waste Manag. 2020, 104, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Gecol, H.; Scamehorn, J.F.; Christian, S.D.; Grady, B.P.; Riddell, F. Use of surfactants to remove water based inks from plastic films. Colloids Surf. A Physicochem. Eng. Asp. 2001, 189, 55–64. [Google Scholar] [CrossRef]
- Gecol, H.; Scamehorn, J.F.; Christian, S.D.; Riddell, F.E. Use of surfactants to remove solvent-based inks from plastic films. Colloid Polym. Sci. 2003, 281, 1172–1177. [Google Scholar] [CrossRef]
- Genuino, H.C.; Ruiz, M.P.; Heeres, H.J.; Kersten, S.R. Pyrolysis of mixed plastic waste (DKR-350): Effect of washing pre-treatment and fate of chlorine. Fuel Process. Technol. 2022, 233. [Google Scholar] [CrossRef]
- Gecol, H.; Scamehorn, J.F.; Christian, S.D.; Grady, B.P.; Riddell, F.E. Deinking of water-based ink printing from plastic film using nonionic surfactants. J. Surfactants Deterg. 2002, 5, 363–374. [Google Scholar] [CrossRef]
- Chotipong, A.; Scamehorn, J.F.; Rirksomboon, T.; Chavadej, S.; Supaphol, P. Removal of solvent-based ink from printed surface of high-density polyethylene bottles by alkyltrimethylammonium bromides: Effects of pH, temperature, and salinity. Colloids Surf. A Physicochem. Eng. Asp. 2007, 297, 163–171. [Google Scholar] [CrossRef]
- Ye, X.; Wu, Z.; Wang, M.; Lv, Y.; Huang, X.; Liu, Y.; Lin, C. Effectively remove printing ink from plastic surface over quaternary ammonium modified waste cooking oil. Environ. Technol. 2022, 1–21. [Google Scholar] [CrossRef]
- Fullana, A.; Lozano, M.A. Method for Removing Ink Printed on Plastic Films. Patent No. EP20130770017 2015.
- Siegwerk. Siegwerk and APK AG Succeed at De-Inking of Plastic Film-Recyclate. 2020. Available online: https://www.siegwerk.com/en/news/press-releases/details/siegwerk-and-apk-ag-succeed-at-de-inking-of-plastic-film-recyclate.html (accessed on 28 August 2022).
- Krevelen, D. Properties of Polymers. Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier Science & Technology: Oxford, UK, 2009. [Google Scholar]
- Thomas, M.; Mittal, K.L. Atmospheric Pressure Plasma Treatment of Polymers. Relevance to Adhesion; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Lindner, M.; Rodler, N.; Jesdinszki, M.; Schmid, M.; Sängerlaub, S. Surface energy of corona treated PP, PE and PET films, its alteration as function of storage time and the effect of various corona dosages on their bond strength after lamination. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, S.L.; Rose, P.W. Plasma surface treatment of plastics to enhance adhesion. Int. J. Adhes. Adhes. 1991, 11, 109–113. [Google Scholar] [CrossRef]
- Kruse, A.; Krüger, G.; Baalmann, A.; Hennemann, O.-D. Surface pre-treatment of plastics for adhesive bonding. J. Adhes. Sci. Technol. 1995, 9, 1611–1621. [Google Scholar] [CrossRef]
- Izdebska, J. 11 Flexographic Printing//Flexographic Printing; Willian Andrew: New York, NY, USA, 2016. [Google Scholar]
- Leach, R.H.; Pierce, R.J.; Hickman, E.P.; Mackenzie, M.J.; Smith, H.G. The Printing Ink Manual; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Pekarovicova, A.; Husovska, V. Printing Ink Formulations; Willian Andrew: New York, NY, USA, 2016. [Google Scholar]
- El-Wahab, H.A.; El-Meligi, G.; Hassaan, M.G.; Kazlauciunas, A.; Lin, L. New water-based copolymer nanoparticles and their use as eco-friendly binders for industry of flexographic ink, part I. Pigment Resin Technol. 2020, 49, 239–248. [Google Scholar] [CrossRef]
- Fink, J.K. Ink Types; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Samorì, C.; Cespi, D.; Blair, P.; Galletti, P.; Malferrari, D.; Passarini, F.; Vassura, I.; Tagliavini, E. Application of switchable hydrophilicity solvents for recycling multilayer packaging materials. Green Chem. 2017, 19, 1714–1720. [Google Scholar] [CrossRef]
- Yousef, S.; Mumladze, T.; Tatariants, M.; Kriūkienė, R.; Makarevicius, V.; Bendikiene, R.; Denafas, G. Cleaner and profitable industrial technology for full recovery of metallic and non-metallic fraction of waste pharmaceutical blisters using switchable hydrophilicity solvents. J. Clean. Prod. 2018, 197, 379–392. [Google Scholar] [CrossRef]
- Mumladze, T.; Yousef, S.; Tatariants, M.; Kriūkienė, R.; Makarevicius, V.; Lukošiūtė, S.-I.; Bendikiene, R.; Denafas, G. Sustainable approach to recycling of multilayer flexible packaging using switchable hydrophilicity solvents. Green Chem. 2018, 20, 3604–3618. [Google Scholar] [CrossRef]
- Ügdüler, S.; de Somer, T.; van Geem, K.M.; de Wilde, J.; Roosen, M.; Deprez, B.; de Meester, S. Analysis of the kinetics, energy balance and carbon footprint of the delamination of multilayer flexible packaging films via carboxylic acids. Resour. Conserv. Recycl. 2022, 181, 106256. [Google Scholar] [CrossRef]
- O’Rourke, G.E.; Houbrechts, M.; Nees, M.; Roosen, M.; De Meester, S.; De Vos, D.E. Delamination of polyamide/polyolefin multilayer films by selective glycolysis of polyurethane adhesive. Green Chem. 2022, 24, 6867–6878. [Google Scholar] [CrossRef]
- Wyatt, F. Flexographicinks; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- PubChem, Compound Summary—Cetrimonium Bromide. Available online: https://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?cid=5974&t=s (accessed on 10 June 2022).
- EPA. Substance Registry Services (SRS) Login for EPA & Partners Oxirane, Methyl-, Polymer with Oxirane, Mono (3,5,5-Trimethylhexyl) Ether. Available online: https://sor.epa.gov/sor_internet/registry/substreg/searchandretrieve/substancesearch/search.do?details=displayDetails&selectedSubstanceId=81194 (accessed on 10 June 2022).
- Wunderlich, B. Thermal Analysis of Polymeric Materials; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- ISO 527-1:2012-06; Kunststoffe—Bestimmung der Zugeigenschaften—Teil_1: Allgemeine Grundsätze (ISO_527-1:2012). Deutsche Fassung EN_ISO_527-1:2012. Beuth Verlag GmbH: Berlin, Germany, 2012.
- Demets, R.; Van Kets, K.; Huysveld, S.; Dewulf, J.; De Meester, S.; Ragaert, K. Addressing the complex challenge of understanding and quantifying substitutability for recycled plastics. Resour. Conserv. Recycl. 2021, 174, 105826. [Google Scholar] [CrossRef]
- Jin, H.; Gonzalez-Gutierrez, J.; Oblak, P.; Zupančič, B.; Emri, I. The effect of extensive mechanical recycling on the properties of low density polyethylene. Polym. Degrad. Stab. 2012, 97, 2262–2272. [Google Scholar] [CrossRef]
- Desai, V.; Shenoy, M.A.; Gogate, P.R. Ultrasonic degradation of low-density polyethylene. Chem. Eng. Process. Process Intensif. 2008, 47, 1451–1455. [Google Scholar] [CrossRef]
- Żołek-Tryznowska, Z. Additives for Ink Manufacture; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Gilbert, M. Brydson’s Plastics Materials; Butterworth-Heinemann: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany, 2017. [Google Scholar]
- Romisuhani, A.; Salmah, H.; Akmal, H. Tensile properties of low density polypropylene (LDPE)/palm kernel shell (PKS) biocomposites: The effect of acrylic acid (AA). IOP Conf. Ser. Mater. Sci. Eng. 2010, 11, 12001. [Google Scholar] [CrossRef]










| Category | Origin | Source |
|---|---|---|
| Intentionally added substances (IAS) | Additives | [27,28,29,30] |
| Processing agents | [26,31] | |
| Printing ink | [32,33] | |
| Adhesion | [24,26] | |
| Non-intentionally added substances (NIAS) | Degradation of products of IAS | [25,26] |
| Impurities from IAS | [25,31] | |
| Side reaction products | [25] | |
| Contamination from recycling processes | [32] |
| Water-Based Ink System | Solvent-Based Ink System |
|---|---|
| Methyl methacrylate | nitrocellulose (NC) |
| Acrylates, i.e., styrene, butyl acrylates | polyamide (PA) |
| Acrylic and methacrylic acid | polyvinyl butyral (PVB) |
| methacrylates | polyvinyl chloride (PVC) |
| acrylic acid | polyurethane (PU) |
| Sample Name | Surfactant | pH |
|---|---|---|
| S1 | CTAB, 1 wt.% | 12 |
| S2 | CTAB, 1 wt.% | 10 |
| S3 | oxirane, methyl-, polymer with oxirane, iso-alkyl alcohol 1 wt.% | 10 |
| S4 | oxirane, methyl-, polymer with oxirane, iso-alkyl alcohol 1 wt.% | 11 |
| S5 | oxirane, methyl-, polymer with oxirane, iso-alkyl alcohol 1 wt.% | 12 |
| S6 | oxirane, methyl-, polymer with oxirane, iso-alkyl alcohol 1 wt.% plus 1 wt.% macroemulsifier DA850 | 10 |
| S7 | oxirane, methyl-, polymer with oxirane, iso-alkyl alcohol 1 wt.% plus 1 wt.% macroemulsifier DA850 | 11 |
| S8 | oxirane, methyl-, polymer with oxirane, iso-alkyl alcohol 1 wt.% plus 1 wt.% macroemulsifier DA850 | 12 |
| Sample Name | Surfactant | T (°C) | pH |
|---|---|---|---|
| SU **-1 | CTAB, 1 wt.% | 40 | 10 |
| SU-2 | CTAB, 1 wt.% | 40 | 12 |
| SU-3 | oxirane, methyl-, polymer with oxirane, iso-alkyl alcohol 1 wt.%plus 1 wt.% macroemulsifier DA850 | 40 | 10 |
| SU-4 | oxirane, methyl-, polymer with oxirane, iso-alkyl alcohol 1 wt.%plus 1 wt.% macroemulsifier DA850 | 40 | 12 |
| P * | - | 80 | 12.7 (RT) |
| NP * | - | 80 | 12.7 (RT) |
| Recycling Quality (RQ) | Parameter | Range/Equation for RQ | ||||
|---|---|---|---|---|---|---|
| RQproc | MFI | 0–0.1875 RQ = 0 | 0.1875–0.25 RQ = 16 * MFI−3 | 0.25–4 RQ = 1 | 4–5 RQ = −MFI + 5 | >5 RQ = 0 |
| RQmech | E-Modulus (E) | 0–89.6 RQ = 0.011161E | 89.6–300 RQ = 1 | 300–600 RQ = −0.00333 + 2 | >600 RQ = 0 | |
| Yield strength (ơy) | 0–10 RQ = 0.1 ơy | >10 RQ = 1 | ||||
| Strain at break (εb) | 0–300 RQ = 0.00333 εb | >300 RQ = 1 | ||||
| Sample Names | Tm (°C) | Tc (°C) | Xc (%) |
|---|---|---|---|
| SU-1 | 112.12 | 126.17 | 44.92 |
| SU-2 | 111.15 | 126.58 | 44.63 |
| SU-3 | 110.71 | 127.38 | 50.14 |
| SU-4 | 112.45 | 125.44 | 49.72 |
| NP | 111.15 | 126.58 | 46.52 |
| P | 110.29 | 124.16 | 40.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Kim, Y.; Chong, Z.K.; Alassali, A.; Chacon, J.P.; Gottschalk, D.; Kitzberger, M.; Kuchta, K. Quality Changes of Low-Density Polyethylene (LDPE) Recyclates from the Pretreatment Process with a Cationic Surfactant and a Nonionic Surfactant as Cleaning Agents Upstream of Extrusion. Processes 2022, 10, 2174. https://doi.org/10.3390/pr10112174
Guo J, Kim Y, Chong ZK, Alassali A, Chacon JP, Gottschalk D, Kitzberger M, Kuchta K. Quality Changes of Low-Density Polyethylene (LDPE) Recyclates from the Pretreatment Process with a Cationic Surfactant and a Nonionic Surfactant as Cleaning Agents Upstream of Extrusion. Processes. 2022; 10(11):2174. https://doi.org/10.3390/pr10112174
Chicago/Turabian StyleGuo, Jinyang, Youngju Kim, Zhi Kai Chong, Ayah Alassali, Jose Pablo Chacon, Dieter Gottschalk, Magdalena Kitzberger, and Kerstin Kuchta. 2022. "Quality Changes of Low-Density Polyethylene (LDPE) Recyclates from the Pretreatment Process with a Cationic Surfactant and a Nonionic Surfactant as Cleaning Agents Upstream of Extrusion" Processes 10, no. 11: 2174. https://doi.org/10.3390/pr10112174