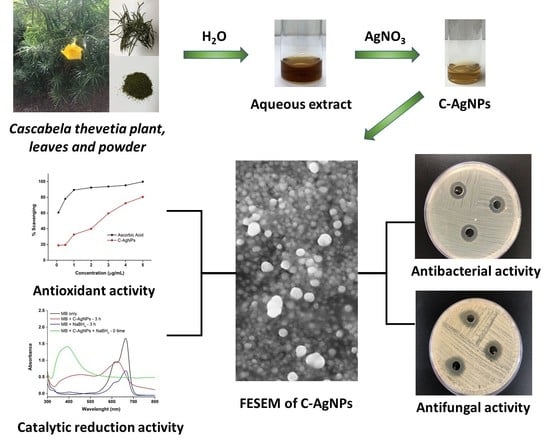
Excellent Antimicrobial, Antioxidant, and Catalytic Activities of Medicinal Plant Aqueous Leaf Extract Derived Silver Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Collection of Cascabela thevetia Leaves
2.2. Preparation of Aqueous Extract
2.3. Synthesis of C-AgNPs
2.4. Characterization of C-AgNPs
2.5. Antibacterial Activity Assessment of C-AgNPs
2.6. Minimum Inhibitory Concentration Test
2.7. Bacterial Growth Study
2.8. Studies of Antifungal Activity
2.9. Antioxidant Activity Test
2.10. Catalytic Reduction Experiment
2.11. Statistical Analysis
3. Results and Discussion
3.1. Color Transformation and UV-Visible Study of C-AgNPs
3.2. HPLC Analysis of Cascabela thevetia Aqueous Leaf Extract
3.3. FTIR Spectra of the Prepared C-AgNPs
3.4. Synthesis Mechanism of C-AgNPs
3.5. X-ray Diffraction Pattern of C-AgNPs
3.6. FESEM Micrographs of C-AgNPs
3.7. TEM Analysis of C-AgNPs
3.8. Antimicrobial Activity of C-AgNPs
3.9. MICs of C-AgNPs
3.10. Bacterial Growth Curves
3.11. Antioxidant Activity Test
3.12. Catalytic Reduction of Textile Dyes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durán, N.; Durán, M.; De Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed.: Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Al-Otibi, F.; Alkhudhair, S.K.; Alharbi, R.I.; Al-Askar, A.A.; Aljowaie, R.M.; Al-Shehri, S. The antimicrobial activities of silver nanoparticles from aqueous extract of grape seeds against pathogenic bacteria and fungi. Molecules 2021, 26, 6081. [Google Scholar] [CrossRef] [PubMed]
- Urnukhsaikhan, E.; Bold, B.E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef] [PubMed]
- Chahar, V.; Sharma, B.; Shukla, G.; Srivastava, A.; Bhatnagar, A. Study of antimicrobial activity of silver nanoparticles synthesized using green and chemical approach. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 149–155. [Google Scholar] [CrossRef]
- Shalaby, T.I.; Mahmoud, O.A.; El Batouti, G.A.; Ibrahim, E.E. Green synthesis of silver nanoparticles: Synthesis, characterization and antibacterial activity. Nanosci. Nanotechnol. 2015, 5, 23–29. [Google Scholar]
- Sreelekha, E.; George, B.; Shyam, A.; Sajina, N.; Mathew, B. A comparative study on the synthesis, characterization, and antioxidant activity of green and chemically synthesized silver nanoparticles. BioNanoScience 2021, 11, 489–496. [Google Scholar] [CrossRef]
- Guan, Z.; Ying, S.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar]
- Patra, J.K.; Baek, K.H. Green nanobiotechnology: Factors affecting synthesis and characterization techniques. J. Nanomater. 2014, 2014, 417305. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Bio. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Z.; Chen, J.; Luan, M. Metabolomic Profile and Antibacterial Bioactivity of Akebia trifoliata (Thunb.) Koidz Pericarp Extract. Processes 2022, 10, 1394. [Google Scholar] [CrossRef]
- Pradeep, M.; Kruszka, D.; Kachlicki, P.; Mondal, D.; Franklin, G. Uncovering the phytochemical basis and the mechanism of plant extract-mediated eco-friendly synthesis of silver nanoparticles using ultra-performance liquid chromatography coupled with a photodiode array and high-resolution mass spectrometry. ACS Sustain. Chem. Eng. 2021, 10, 562–571. [Google Scholar] [CrossRef]
- Salih, A.M.; Al-Qurainy, F.; Khan, S.; Tarroum, M.; Nadeem, M.; Shaikhaldein, H.O.; Gaafar, A.-R.Z.; Alfarraj, N.S. Biosynthesis of zinc oxide nanoparticles using Phoenix dactylifera and their effect on biomass and phytochemical compounds in Juniperus procera. Sci. Rep. 2021, 11, 19136. [Google Scholar] [CrossRef]
- Tomilova, S.V.; Kitashov, A.V.; Nosov, A.M. Cardiac Glycosides: Distribution, Properties and Specificity of Formation in Plant Cell and Organ Cultures In Vitro. Russ. J. Plant Physiol. 2022, 69, 41. [Google Scholar] [CrossRef]
- González-Stuart, A.E.; Rivera, J.O. Herbal weight loss supplements: From dubious efficacy to direct toxicity. In Dietary Interventions in Liver Disease; Academic Press: Cambridge, MA, USA, 2019; pp. 175–181. [Google Scholar]
- Lavecchia, T.; Rea, G.; Antonacci, A.; Giardi, M.T. Healthy and adverse effects of plant-derived functional metabolites: The need of revealing their content and bioactivity in a complex food matrix. Crit. Rev. Food Sci. Nutr. 2013, 53, 198–213. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Antimicrobial activities and phytochemical profiles of endemic medicinal plants of Mauritius. Pharm. Biol. 2005, 43, 237–242. [Google Scholar] [CrossRef]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, D.; Nathan, S.; Suresh, T.; Perumalsamy, P.L. Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine. J. Ethnopharmacol. 2001, 74, 217–220. [Google Scholar] [CrossRef]
- Ushimaru, P.I.; Silva, M.T.N.D.; Di Stasi, L.C.; Barbosa, L.; Fernandes Junior, A. Antibacterial activity of medicinal plant extracts. Braz. J. Microbiol. 2007, 38, 717–719. [Google Scholar] [CrossRef]
- Marasini, B.P.; Baral, P.; Aryal, P.; Ghimire, K.R.; Neupane, S.; Dahal, N.; Singh, A.; Ghimire, L.; Shrestha, K. Evaluation of antibacterial activity of some traditionally used medicinal plants against human pathogenic bacteria. BioMed Res. Int. 2015, 2015, 265425. [Google Scholar] [CrossRef]
- González-Stuart, A. Dangerous herbal weight-loss supplements. In Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease, 1st ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2013; p. 119. [Google Scholar]
- Kohls, S.; Scholz-Böttcher, B.M.; Teske, J.; Zark, P.; Rullkötter, J. Cardiac glycosides from Yellow Oleander (Thevetia peruviana) seeds. Phytochemistry 2012, 75, 114–127. [Google Scholar] [CrossRef]
- Zibbu, G.; Batra, A. Thevetia peruviana (Pers.) Schum.: A plant with enormous therapeutic potential. J. Pharm. Res. 2011, 4, 4461–4464. [Google Scholar]
- Falih, H.Y.; Nasser, N.M.; Jasiem, T.M. Pharmacognostical Study and Antibacterial Activity of Cardio Active Glycoside of Iraqi Yellow Oleander (Thevetia Peruviana L.). Indian J. Public Health Res. Dev. 2019, 10, 3128. [Google Scholar] [CrossRef]
- Pillay, V.V.; Sasidharan, A. Oleander and datura poisoning: An update. Indian J. Crit. Care Med. 2019, 23, S250–S255. [Google Scholar] [CrossRef]
- Yamane, H.; Konno, K.; Sabelis, M.; Takabayashi, J.; Sassa, T.; Oikawa, H. Chemical defence and toxins of plants. In Comprehensive Natural Products II: Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 4, pp. 339–385. [Google Scholar]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef]
- Bonigala, B.; YVV, A.K.; Kondabolu, U.L.; Poda, S. Ecofriendly Synthesis of Silver Nanoparticles by using Indian Plants and Screening their Catalytic Activity. Curr. Trends Biotechnol. Pharm. 2016, 10, 316–323. [Google Scholar]
- Bonigala, B.; Mangamuri, U.K.; Anuhya, G.; Saraswathi, Y.Y.; Rao, K.R.S.; Poda, S. Green Synthesis of Silver Nanoparticles using two Apocyanaceae plants and Screening for their Catalytic activity. Curr. Trends Biotechnol. Pharm. 2017, 11, 84–90. [Google Scholar]
- Oluwaniyi, O.O.; Adegoke, H.I.; Adesuji, E.T.; Alabi, A.B.; Bodede, S.O.; Labulo, A.H.; Oseghale, C.O. Biosynthesis of silver nanoparticles using aqueous leaf extract of Thevetia peruviana Juss and its antimicrobial activities. Appl. Nanosci. 2016, 6, 903–912. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Stobiecki, M. Application of mass spectrometry for identification and structural studies of flavonoid glycosides. Phytochemistry 2000, 54, 237–256. [Google Scholar] [CrossRef]
- Morehead, M.S.; Scarbrough, C. Emergence of global antibiotic resistance. Prim. Care Clin. Off. Pract. 2018, 45, 467–484. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Qadri, H.; Shah, A.H.; Andrabi, S.M.; Alshehri, B.; Almilaibary, A.; Mir, M.A. Natural Products And Their Semi-synthetic Derivatives Against Antimicrobial-resistant Human Pathogenic Bacteria and Fungi. Saudi J. Biol. Sci. 2022, 29, 103376. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infec. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Wahab, S.; Khan, T.; Adil, M.; Khan, A. Mechanistic aspects of plant-based silver nanoparticles against multi-drug resistant bacteria. Heliyon 2021, 7, e07448. [Google Scholar] [CrossRef]
- Skóra, B.; Krajewska, U.; Nowak, A.; Dziedzic, A.; Barylyak, A.; Kus-Liśkiewicz, M. Noncytotoxic silver nanoparticles as a new antimicrobial strategy. Sci. Rep. 2021, 11, 13451. [Google Scholar] [CrossRef]
- Chen, H.; Zuo, Y.; Deng, Y. Separation and determination of flavonoids and other phenolic compounds in cranberry juice by high-performance liquid chromatography. J. Chromatogr. 2001, 913, 387–395. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Lewis, B.A.; Brown, D.L.; Rodriguez, E.; Obendorf, R.L. Characterisation of phenolics by LC–UV/Vis, LC–MS/MS and sugars by GC in Melicoccus bijugatus Jacq. ‘Montgomery’ fruits. Food Chem. 2008, 111, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Hao, Z.; Li, M. Isolation and structure identification of flavonoids. In Flavonoids, From Biosynthesis to Human Health; Justino, G.C., Ed.; IntechOpen: London, UK, 2017; pp. 17–43. [Google Scholar]
- El-Sawi, S.A.; Maamoun, A.A.; Salama, A.H.; Farghaly, A.A. Chemical profiling of Thevetia peruviana leaves cytotoxic active extracts enhanced by microemulsion formulation. Bull. Natl. Res. Cent. 2020, 44, 93. [Google Scholar] [CrossRef]
- Vankar, P.S.; Shukla, D. Spectrum of colors from reseda luteola and other natural yellow dyes. J. Tex. Eng. Fash. Technol. 2018, 4, 107–120. [Google Scholar] [CrossRef]
- Thilagavathi, R.; Kavitha, H.P.; Venkatraman, B.R. Isolation, characterization and anti-inflammatory property of Thevetia peruviana. J. Chem. 2010, 7, 1584–1590. [Google Scholar]
- Durán, M.D.L.; Zabala, M.E.A.; Londoño, G.A.C. Optimization of Flavonoid Production in Plant Cell Culture of Thevetia peruviana Elicited with Methyl Jasmonate and Salicylic Acid. Braz. Arch. Biol. Technol. 2021, 64, 2021210022. [Google Scholar] [CrossRef]
- Pinho, E.; Soares, G.; Henriques, M. Cyclodextrin modulation of gallic acid in vitro antibacterial activity. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 205–214. [Google Scholar] [CrossRef]
- Badhani, B.; Kakkar, R. DFT study of structural and electronic properties of gallic acid and its anions in gas phase and in aqueous solution. Struct. Chem. 2017, 28, 1789–1802. [Google Scholar] [CrossRef]
- Raza, M.A.; Shahwar, D.; Khan, T. Radical scavenging, proteases activities, and phenolics composition of bark extracts from 21 medicinal plants. J. Chem. 2015, 2015, 951840. [Google Scholar]
- Ramachandran, S.; Asokkumar, K.; Maheswari, M.U.; Ravi, T.K.; Sivashanmugam, A.T.; Saravanan, S.; Rajasekaran, A.; Dharman, J. Investigation of antidiabetic, antihyperlipidemic, and in vivo antioxidant properties of Sphaeranthus indicus Linn. in type 1 diabetic rats: An identification of possible biomarkers. Evid. Based Complementary Altern. Med. 2011, 2011, 571721. [Google Scholar] [CrossRef]
- Esmaeili, A.H.; Moghaddam, A.H.; Chaichi, M.J. Identification, determination, and study of antioxidative activities of hesperetin and gallic acid in hydro-alcoholic extract from flowers of Eriobotrya japonica (Lindl.). Avicenna J. Phytomedicine 2014, 4, 260. [Google Scholar]
- Manthey, J.A. Fourier transform infrared spectroscopic analysis of the polymethoxylated flavone content of orange oil residues. J. Agric. Food Chem. 2006, 54, 3215–3218. [Google Scholar] [CrossRef]
- Patle, T.K.; Shrivas, K.; Kurrey, R.; Upadhyay, S.; Jangde, R.; Chauhan, R. Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV–vis and FTIR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 242, 118717. [Google Scholar] [CrossRef]
- Chahardoli, A.; Hajmomeni, P.; Ghowsi, M.; Qalekhani, F.; Shokoohinia, Y.; Fattahi, A. Optimization of Quercetin-Assisted Silver Nanoparticles Synthesis and Evaluation of Their Hemocompatibility, Antioxidant, Anti-Inflammatory, and Antibacterial effects. Glob. Chall. 2021, 5, 2100075. [Google Scholar] [CrossRef]
- Bashami, R.M.; Soomro, M.T.; Khan, A.N.; Aazam, E.S.; Ismail, I.M.; El-Shahawi, M.S. A highly conductive thin film composite based on silver nanoparticles and malic acid for selective electrochemical sensing of trichloroacetic acid. Anal. Chim. Acta 2018, 1036, 33–48. [Google Scholar] [CrossRef]
- Yazdanshenas, M.E.; Mohammad, S.-K. The effect of alkali pre-treatment on formation and adsorption of silver nanoparticles on cotton surface. Fibers Polym. 2012, 13, 1170–1178. [Google Scholar] [CrossRef]
- Tan, M.; Choi, Y.; Kim, J.; Kim, J.-H.; Fromm, K.M. Polyaspartamide functionalized catechol-based hydrogels embedded with silver nanoparticles for antimicrobial properties. Polymers 2018, 10, 1188. [Google Scholar] [CrossRef]
- Naseer, F.; Zahir, E.; Danish, E.Y.; Gull, M.; Noman, S.; Soomro, M.T. Superior antibacterial activity of reduced graphene oxide upon decoration with iron oxide nanorods. J. Environ. Chem. Eng. 2020, 8, 104424. [Google Scholar] [CrossRef]
- Brandelli, A. The interaction of nanostructured antimicrobials with biological systems: Cellular uptake, trafficking and potential toxicity. Food Sci. Hum. Wellness 2020, 9, 8–20. [Google Scholar] [CrossRef]
- Vijayakumar, G.; Kesavan, H.; Kannan, A.; Arulanandam, D.; Kim, J.H.; Kim, K.J.; Sogn, H.J.; Kim, H.J.; Rangarajulu, S.K. Phytosynthesis of copper nanoparticles using extracts of spices and their antibacterial properties. Processes 2021, 9, 1341. [Google Scholar] [CrossRef]
- Anand, U.; Carpena, M.; Kowalska-Góralska, M.; Garcia-Perez, P.; Sunita, K.; Bontempi, E.; Dey, A.; Prieto, M.A.; Prockow, J.; Simal-Gandara, J. Safer plant-based nanoparticles for combating antibiotic resistance in bacteria: A comprehensive review on its potential applications, recent advances, and future perspective. Sci. Total Environ. 2022, 821, 153472. [Google Scholar] [CrossRef]
- Al-Qasmi, N.; Soomro, M.T.; Aslam, M.; Rehman, A.U.; Ali, S.; Danish, E.Y.; Ismail, I.M.I.; Hameed, A. The efficacy of the ZnO:α-Fe2O3 composites modified carbon paste electrode for the sensitive electrochemical detection of loperamide: A detailed investigation. J. Electroanal. Chem. 2016, 783, 112–124. [Google Scholar] [CrossRef]
- Aslam, M.; Qamar, M.T.; Ali, S.; Rehman, A.U.; Soomro, M.T.; Ahmed, I.; Ismail, I.M.I.; Hameed, A. Evaluation of SnO2 for sunlight photocatalytic decontamination of water. J. Environ. Manag. 2018, 217, 805–814. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Antibacterial Effect of Silver Nanoparticles Is Stronger If the Production Host and the Targeted Pathogen Are Closely Related. Biomedicines 2022, 10, 628. [Google Scholar] [CrossRef]
- Alahmad, A.; Al-Zereini, W.A.; Hijazin, T.J.; Al-Madanat, O.Y.; Alghoraibi, I.; Al-Qaralleh, O.; Al-Qaraleh, S.; Feldhoff, A.; Walter, J.-G.; Scheper, T. Green Synthesis of Silver Nanoparticles Using Hypericum perforatum L. Aqueous Extract with the Evaluation of Its Antibacterial Activity against Clinical and Food Pathogens. Pharmaceutics 2022, 14, 1104. [Google Scholar] [CrossRef]
- Naveed, M.; Batool, H.; Javed, A.; Makhdoom, S.I.; Aziz, T.; Mohamed, A.A.; Sameeh, M.Y.; Alruways, M.W.; Dablool, A.S.; Almalki, A.A.; et al. Characterization and Evaluation of the Antioxidant, Antidiabetic, Anti-Inflammatory, and Cytotoxic Activities of Silver Nanoparticles Synthesized Using Brachychiton populneus Leaf Extract. Processes 2022, 10, 1521. [Google Scholar] [CrossRef]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of antifungal drug resistance. Cold Spring Harb. Perspect. Med. 2015, 5, a019752. [Google Scholar] [CrossRef]
- Kumari, M.; Giri, V.P.; Pandey, S.; Kumar, M.; Katiyar, R.; Nautiyal, C.S.; Mishra, A. An insight into the mechanism of antifungal activity of biogenic nanoparticles than their chemical counterparts. Pestic. Biochem. Physiol. 2019, 157, 45–52. [Google Scholar] [CrossRef]
- Mansoor, S.; Zahoor, I.; Baba, T.R.; Padder, S.A.; Bhat, Z.A.; Koul, A.M.; Jiang, L. Fabrication of silver nanoparticles against fungal pathogens. Front. Nanotech. 2021, 3, 679358. [Google Scholar] [CrossRef]
- Padhi, S.; Behera, A. Silver-based nanostructures as antifungal agents: Mechanisms and applications. In Silver Nanomaterials for Agri-Food Applications; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 17–38. [Google Scholar]
- de Torre, M.P.; Cavero, R.Y.; Calvo, M.I.; Vizmanos, J.L. A simple and a reliable method to quantify antioxidant activity in vivo. Antioxidants 2019, 8, 142. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, M.I.; Khan, S.B.; Akhtar, K.; Khan, M.A.; Asiri, A.M. Catalytic reduction of picric acid, nitrophenols and organic azo dyes via green synthesized plant supported Ag nanoparticles. J. Mol. Liq. 2018, 268, 87–101. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Lee, Y.; Kim, M.J.; Ahn, C.W. Biomimetic synthesis of silver nanoparticles using Syzygium aromaticum (clove) extract: Catalytic and antimicrobial effects. Appl. Organomet. Chem. 2019, 33, e4867. [Google Scholar] [CrossRef]
- Hu, M.; Yan, X.; Hu, X.; Feng, R.; Zhou, M. Synthesis of silver decorated silica nanoparticles with rough surfaces as adsorbent and catalyst for methylene blue removal. J. Sol-Gel Sci. Technol. 2019, 89, 754–763. [Google Scholar] [CrossRef]


















| Microorganisms | Zone of Inhibition (mm) | |||
|---|---|---|---|---|
| Cascabela thevetia Aqueous Leaf Extract | C-AgNPs | |||
| 100 µL | 108 µg/mL | 216 µg/mL | 432 µg/mL | |
| MRSA | - | 14 ± 0.4 | 11 ± 0.1 | 16 ± 0.4 |
| S. aureus | - | 14 ± 0.3 | 15 ± 0.6 | 16 ± 0.5 |
| E. faecalis | - | 12 ± 0.8 | 15 ± 0.4 | 14 ± 0.1 |
| E. coli | - | 12 ± 0.1 | 13 ± 0.1 | 14 ± 0.3 |
| S. tyhpimurium | - | 13 ± 0.7 | 15 ± 0.2 | 15 ± 0.7 |
| K. pheumoniae | - | 12 ± 0.1 | 13 ± 0.9 | 14 ± 0.3 |
| P. aeruginosa | - | 13 ± 0.2 | 13 ± 0.1 | 13 ± 0.6 |
| C. albicans | - | 11 ± 0.8 | 12 ± 0.4 | 18 ± 0.9 |
| C. parapsilosis | - | 15 ± 0.7 | 16 ± 0.6 | 19 ± 0.4 |
| Bacterial Pathogens | C-AgNPs (µg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 54 | 27 | 13.5 | 6.75 | 3.38 | 1.69 | 0.84 | 0.42 | 0.21 | 0.105 | |
| S. aureus | − | − | + | + | + | + | + | + | + | + |
| E. faecalis | − | − | − | + | + | + | + | + | + | + |
| E. coli | − | − | + | + | + | + | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.N.; Ali Aldowairy, N.N.; Saad Alorfi, H.S.; Aslam, M.; Bawazir, W.A.; Hameed, A.; Soomro, M.T. Excellent Antimicrobial, Antioxidant, and Catalytic Activities of Medicinal Plant Aqueous Leaf Extract Derived Silver Nanoparticles. Processes 2022, 10, 1949. https://doi.org/10.3390/pr10101949
Khan AN, Ali Aldowairy NN, Saad Alorfi HS, Aslam M, Bawazir WA, Hameed A, Soomro MT. Excellent Antimicrobial, Antioxidant, and Catalytic Activities of Medicinal Plant Aqueous Leaf Extract Derived Silver Nanoparticles. Processes. 2022; 10(10):1949. https://doi.org/10.3390/pr10101949
Chicago/Turabian StyleKhan, Amna Nisar, Najla Nader Ali Aldowairy, Hajer Saed Saad Alorfi, Mohammad Aslam, Wafa AbuBaker Bawazir, Abdul Hameed, and Muhammad Tahir Soomro. 2022. "Excellent Antimicrobial, Antioxidant, and Catalytic Activities of Medicinal Plant Aqueous Leaf Extract Derived Silver Nanoparticles" Processes 10, no. 10: 1949. https://doi.org/10.3390/pr10101949