Environmental Risk Factors for Childhood Inflammatory Bowel Diseases: A Multicenter Case-Control Study
Abstract
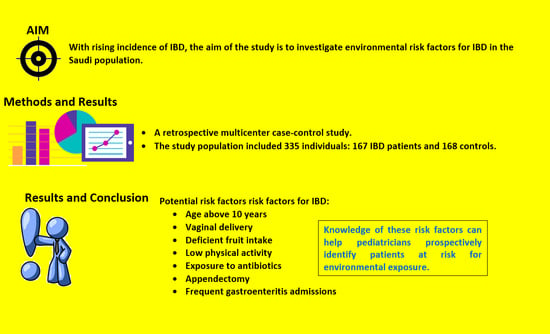
:1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Risk Factors for IBD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ, A.; Latz, E. The Western lifestyle has lasting effects on metaflammation. Nat. Rev. Immunol. 2019, 19, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.P.C.; Gomes, C.; Torres, J. Familial and ethnic risk in inflammatory bowel disease. Ann. Gastroenterol. 2018, 31, 14–23. [Google Scholar] [CrossRef]
- Lindberg, E.; Tysk, C.; Andersson, K.; Järnerot, G. Smoking and inflammatory bowel disease. A case control study. Gut 1988, 29, 352–357. [Google Scholar] [CrossRef] [Green Version]
- Salgado, V.C.; Luiz, R.R.; Boéchat, N.L.; Leão, I.S.; do Carmo Schorr, B.; Parente, J.M.; Lima, D.C.; Júnior, E.S.; Silva, G.O.; Almeida, N.P.; et al. Risk factors associated with inflammatory bowel disease:A multicenter case-control study in Brazil. World J. Gastroenterol. 2020, 7, 3611–3624. [Google Scholar] [CrossRef]
- Canova, C.; Ludvigsson, J.F.; Di Domenicantonio, R.; Zanier, L.; Barbiellini Amidei, C.; Zingone, F. Perinatal and antibiotic exposures and the risk of developing childhood-onset inflammatory bowel disease: A nested case-control study based on a population-based birth cohort. Int. J. Environ. Res. Public Health 2020, 17, 2409. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Lochhead, P.; Ko, Y.; Claggett, B.; Leong, R.W.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Breastfeeding and the risk of Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Therapeutics 2017, 46, 780–789. [Google Scholar] [CrossRef]
- Algethmi, W.; Baumann, C.; Alnajjar, W.; Sroji, A.; Alsahafi, M.; Jawa, H.; Mosli, M. Environmental exposures and the risk of inflammatory bowel disease: A case-control study from Saudi Arabia. Eur. J. Gastroenterol. Hepatol. 2020, 32, 358–364. [Google Scholar] [CrossRef]
- El Mouzan, M.I.; Saadah, O.; Al-Saleem, K.; Al Edreesi, M.; Hasosah, M.; Alanazi, A.; Al Mofarreh, M.; Asery, A.; Al Qourain, A.; Nouli, K.; et al. Incidence of pediatric inflammatory bowel disease in Saudi Arabia: A multicenter national study. Inflamm. Bowel Dis. 2014, 20, 1085–1090. [Google Scholar] [CrossRef]
- Eri, R.; Yang, H.; Barmada, M.M.; Cavanaugh, J.A.; Annese, V.; Brant, S.R.; Cho, J.H.; Duerr, R.; Hugot, J.-P.; McGovern, D.P.; et al. The IBD international genetics consortium provides further evidence for linkage to IBD4 and shows gene-environment interaction. Inflamm Bowel Dis. 2005, 11, 1–7. [Google Scholar]
- Buderus, S.; Scholz, D.; Behrens, R.; Classen, M.; De Laffolie, J.; Keller, K.M.; Koletzko, S. Inflammatory bowel disease in pediatric patients: Characteristics of newly diagnosed patients from the CEDATA-GPGE Registry. Dtsch. Arztebl. Int. 2015, 112, 121–127. [Google Scholar]
- Bruce, A.; Black, M.; Bhattacharya, S. Mode of delivery and risk of inflammatory bowel disease in the offspring: Systematic review and meta-analysis of observational studies. Inflamm. Bowel Dis. 2014, 20, 1217–1226. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [Green Version]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology 2019, 157, 647–659.e4. [Google Scholar] [CrossRef] [Green Version]
- Radford-Smith, G.L. What is the importance of appendectomy in the natural history of IBD? Inflamm. Bowel Dis. 2008, 14 (Suppl. 2), S72–S74. [Google Scholar] [CrossRef]
- Shouval, D.S.; Rufo, P.A. The Role of Environmental Factors in the Pathogenesis of Inflammatory Bowel Diseases: A Review. JAMA Pediatr. 2017, 171, 999–1005. [Google Scholar] [CrossRef]
- Sun, W.; Han, X.; Wu, S.; Yang, C. Tonsillectomy and the risk of inflammatory bowel disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 1085–1094. [Google Scholar] [CrossRef]
- Burisch, J.; Pedersen, N.; Cukovic-Cavka, S.; Turk, N.; Kaimakliotis, I.; Duricova, D.; Munkholm, P. Environmental factors in a population-based inception cohort of inflammatory bowel disease patients in Europe—An ECCO-EpiCom study. J. Crohns. Colitis 2014, 8, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2014, 63, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Geerling, B.J.; Dagnelie, P.C.; Badart-Smook, A.; Russel, M.G.; Stockbrügger, R.W.; Brummer, R.J. Diet as a risk factor for the development of ulcerative colitis. Am. J. Gastroenterol. 2000, 95, 1008–1013. [Google Scholar] [CrossRef]
- Sakamoto, N.; Kono, S.; Wakai, K.; Epidemiology Group of the Research Committee on Inflammatory Bowel Disease in Japan. Dietary risk factors for inflammatory bowel disease: A multicenter case-control study in Japan. Inflamm. Bowel Dis. 2005, 11, 154–163. [Google Scholar] [CrossRef]
- Galvez, J.; Rodriguez-Cabezas, M.E.; Zarzuelo, A. Effects of dietary fiber on inflammatory bowel disease. Mol. Nutr. Food Res. 2005, 49, 601–608. [Google Scholar] [CrossRef]
- Kugathasan, S.; Nebel, J.; Skelton, J.A.; Markowitz, J.; Keljo, D.; Rosh, J.; Leleiko, N.; Mack, D.; Griffiths, A.; Bousvaros, A.; et al. Body mass index in children with newly diagnosed inflammatory bowel disease: Observations from two multicenter North American inception cohorts. J. Pediatr. 2007, 151, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Long, M.D.; Crandall, W.V.; Leibowitz, I.H.; Duffy, L.; Del Rosario, F.; Kim, S.C.; Integlia, M.J.; Berman, J.; Grunow, J.; Colletti, R.B.; et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 2162–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koloski, N.A.; Bret, L.; Radford-Smith, G. Hygiene hypothesis in inflammatory bowel disease: A critical review of the literature. World J. Gastroenterol. 2008, 14, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.S.; Kroeker, K.; Fedorak, D.; Dieleman, L.; Fedorak, R.N. Prevalence of Epstein-Barr Virus in a population of patients with inflammatory bowel disease: A prospective cohort study. Aliment. Pharm. Ther. 2013, 38, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- García Rodríguez, L.A.; Ruigómez, A.; Panés, J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology 2006, 130, 1588–1594. [Google Scholar] [CrossRef]
- Sajadinejad, M.S.; Asgari, K.; Molavi, H.; Kalantari, M.; Adibi, P. Psychological issues in inflammatory bowel disease: An overview. Gastroenterol. Res. Pract. 2012, 2012, 106502. [Google Scholar] [CrossRef]
- Giannakopoulos, G.; Chouliaras, G.; Margoni, D.; Korlou, S.; Hantzara, V.; Panayotou, I.; Roma, E.; Liakopoulou, M.; Anagnostopoulos, D.C. Stressful life events and psychosocial correlates of pediatric inflammatory bowel disease activity. World J. Psychiatry 2016, 6, 322–328. [Google Scholar] [CrossRef]
- Jakobsen, C.; Paerregaard, A.; Munkholm, P.; Wewer, V. Environmental factors and risk of developing paediatric inflammatory bowel disease: A population based study 2007–2009. J. Crohn’s Colitis 2013, 7, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Hildebrand, H.; Malmborg, P.; Askling, J.; Ekbom, A.; Montgomery, S.M. Early-life exposures associated with antibiotic use and risk of subsequent Crohn’s disease. Scand J. Gastroenterol. 2008, 43, 961–966. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Gatt, K.; Schembri, J.; Katsanos, K.H.; Christodoulou, D.; Karmiris, K.; Kopylov, U.; Pontas, C.; Koutroubakis, I.E.; Foteinogiannopoulou, K.; Fabian, A.; et al. Inflammatory Bowel Disease [IBD] and Physical Activity: A Study on the Impact of Diagnosis on the Level of Exercise amongst Patients with IBD. J. Crohn’s Colitis 2019, 13, 686–692. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Long, M.D.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin. Gastroenterol. Hepatol. 2013, 11, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Mahid, S.S.; Minor, K.S.; Soto, R.E.; Hornung, C.A.; Galandiuk, S. Smoking and inflammatory bowel disease: A meta-analysis. Mayo Clin. Proc. 2006, 81, 1462–1471. [Google Scholar] [CrossRef]
- Allais, L.; Kerckhof, F.-M.; Verschuere, S.; Bracke, K.; De Smet, R.; Laukens, D.; Abbeele, P.V.D.; De Vos, M.; Boon, N.; Brusselle, G.; et al. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ. Microbiol. 2016, 18, 1352–1363. [Google Scholar] [CrossRef]

| n = 335 | % | |
| Diagnosis | ||
| Control | 168 | 50.1 |
| IBD | 167 | 49.9 |
| Age | ||
| <3 years | 21 | 6.3 |
| 3–6 years | 34 | 10.1 |
| 7–10 years | 92 | 27.5 |
| >10 years | 188 | 56.1 |
| Gender (n = 334) | ||
| Male | 172 | 51.5 |
| Female | 162 | 48.5 |
| BMI (n = 334) | ||
| Obesity | 44 | 13.2 |
| No obesity | 290 | 86.8 |
| Housing (n = 334) | ||
| Rural | 41 | 12.3 |
| Urban | 293 | 87.7 |
| Family incomes (n = 333) | ||
| <5000 SR | 53 | 15.9 |
| 5000 SR | 40 | 12.0 |
| >5000 SR | 240 | 72.1 |
| Mother education level | ||
| High | 191 | 57.0 |
| Low | 144 | 43.0 |
| Father education level | ||
| High | 198 | 59.1 |
| Low | 137 | 40.9 |
| Consanguinity 1st degree | ||
| Yes | 133 | 39.7 |
| No | 202 | 60.3 |
| Breastfeeding (n = 333) | ||
| 1 month | 66 | 19.8 |
| <6 months | 95 | 28.5 |
| 6–12 months | 17 | 5.1 |
| >12 months | 155 | 46.5 |
| Type of delivery (n = 333) | ||
| Vaginal | 220 | 66.1 |
| Caesarean | 113 | 33.9 |
| Vaccination | ||
| None | 3 | 0.9 |
| Completed | 302 | 90.1 |
| Partially completed | 30 | 9.0 |
| Parent Smoking (mother) | ||
| Yes | 7 | 2.1 |
| No | 328 | 97.9 |
| Parent Smoking (father) | ||
| Yes | 111 | 33.1 |
| No | 224 | 66.9 |
| Diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | IBD | OR | 95% CI | p | ||||
| n | % | n | % | |||||
| Age (n = 335) | 0.023 | 0.02 | 0.174 | <0.001 * | ||||
| <3 years | 20 | 95.2% | 1 | 4.8% | ||||
| 3–6 years | 32 | 94.1% | 2 | 5.9% | ||||
| 7–10 years | 57 | 62.0% | 35 | 38.0% | ||||
| >10 years | 59 | 31.4% | 129 | 68.6% | ||||
| Gender (n = 334) | 0.714 | 0.464 | 1.099 | 0.125 * | ||||
| Male | 79 | 45.9% | 93 | 54.1% | ||||
| Female | 88 | 54.3% | 74 | 45.7% | ||||
| BMI (n = 334) | 0.655 | 0.344 | 1.247 | 0.196 * | ||||
| Obesity | 18 | 40.9% | 26 | 59.1% | ||||
| No obesity | 149 | 51.4% | 141 | 48.6% | ||||
| Housing (n = 334) | 0.835 | 0.433 | 1.607 | 0.588 * | ||||
| Rural | 19 | 46.3% | 22 | 53.7% | ||||
| Urban | 149 | 50.9% | 144 | 49.1% | ||||
| Family incomes (n = 333) | 1.238 | 0.682 | 2.922 | 0.440 * | ||||
| <5000 SR | 25 | 47.2% | 28 | 52.8% | ||||
| 5000 SR | 17 | 42.5% | 23 | 57.5% | ||||
| >5000 SR | 126 | 52.5% | 114 | 47.5% | ||||
| Mother education level | 1.290 | 0.836 | 1.990 | 0.250 | ||||
| High | 101 | 52.9% | 90 | 47.1% | ||||
| Low | 67 | 46.5% | 77 | 53.5% | ||||
| Father education level | 0.732 | 0.473 | 1.134 | 0.162 | ||||
| High | 93 | 47.0% | 105 | 53.0% | ||||
| Low | 75 | 54.7% | 62 | 45.3% | ||||
| Consanguinity 1st degree | 0.732 | 0.473 | 1.134 | 0.162 | ||||
| Yes | 67 | 50.4% | 66 | 49.6% | ||||
| No | 101 | 50.0% | 101 | 50.0% | ||||
| Breastfeeding (n = 333) | 0.83 | 0.466 | 1.479 | 0.340 * | ||||
| 1 month | 35 | 53.0% | 31 | 47.0% | ||||
| <6 months | 46 | 48.4% | 49 | 51.6% | ||||
| 6 months | 12 | 70.6% | 5 | 29.4% | ||||
| >6 months | 75 | 48.4% | 80 | 51.6% | ||||
| Type of delivery (n = 333) | 0.551 | 0.348 | 0.874 | 0.011 | ||||
| Vaginal | 100 | 45.5% | 120 | 54.5% | ||||
| Caesarean | 68 | 60.2% | 45 | 39.8% | ||||
| Vaccination | cannot be calculated | 0.562 ** | ||||||
| None | 0 | 0.0% | 3 | 100.0% | ||||
| Completely | 150 | 49.7% | 152 | 50.3% | ||||
| Partially complete | 18 | 60.0% | 12 | 40.0% | ||||
| Parent Smoking (mother) | 0.741 | 0.163 | 3.362 | 0.723 ** | ||||
| Yes | 3 | 42.9% | 4 | 57.1% | ||||
| No | 165 | 50.3% | 163 | 49.7% | ||||
| Parent Smoking (father) | 0.914 | 0.580 | 1.441 | 0.699 * | ||||
| Yes | 54 | 48.6% | 57 | 51.4% | ||||
| No | 114 | 50.9% | 110 | 49.1% | ||||
| Diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | IBD | OR | 95% CI | p | ||||
| n | % | n | % | |||||
| Fast food | 1.181 | 0.741 | 4.40 | 0.422 * | ||||
| Daily | 16 | 38.1% | 26 | 61.9% | ||||
| Weekly | 97 | 51.6% | 91 | 48.4% | ||||
| Monthly | 35 | 52.2% | 32 | 47.8% | ||||
| Never | 20 | 52.6% | 18 | 47.4% | ||||
| Fruit intake | 2.572 | 1.597 | 4.142 | <0.001 * | ||||
| Yes | 71 | 65.7% | 37 | 34.3% | ||||
| No | 97 | 42.7% | 130 | 57.3% | ||||
| Food allergy | 0.733 | 0.392 | 1.372 | 0.330 * | ||||
| Yes | 20 | 43.5% | 26 | 56.5% | ||||
| No | 148 | 51.2% | 141 | 48.8% | ||||
| Cow milk allergy | 0.545 | 0.179 | 1.663 | 0.280 * | ||||
| Yes | 5 | 35.7% | 9 | 64.3% | ||||
| No | 161 | 50.5% | 158 | 49.5% | ||||
| Exposure to antibiotics | 2.396 | 1.507 | 3.808 | <0.001 * | ||||
| None | 61 | 38.4% | 98 | 61.6% | ||||
| Monthly | 22 | 64.7% | 12 | 35.3% | ||||
| Yearly | 85 | 59.9% | 57 | 40.1% | ||||
| Water Pollution | 1.200 | 0.359 | 4.011 | 0.767 | ||||
| Yes | 6 | 54.5% | 5 | 45.5% | ||||
| No | 162 | 50.0% | 162 | 50.0% | ||||
| Appendectomy | 2.098 | 1.871 | 2.353 | <0.001 | ||||
| Yes | 0 | 0.0% | 14 | 100.0% | ||||
| No | 168 | 52.3% | 153 | 47.7% | ||||
| Tonsillectomy | 0.653 | 0.312 | 1.370 | 0.257 | ||||
| Yes | 13 | 40.6% | 19 | 59.4% | ||||
| No | 155 | 51.2% | 148 | 48.8% | ||||
| Family Stress, anxiety | 0.259 | 0.155 | 0.433 | <0.001 | ||||
| Yes | 27 | 27.6% | 71 | 72.4% | ||||
| No | 141 | 59.5% | 96 | 40.5% | ||||
| Sleep disturbance | 0.242 | 0.125 | 0.470 | <0.001 | ||||
| Yes | 13 | 23.2% | 43 | 76.8% | ||||
| No | 155 | 55.6% | 124 | 44.4% | ||||
| Physical activity | 2.033 | 1.053 | 3.927 | <0.001 | ||||
| None | 36 | 32.1% | 76 | 67.9% | ||||
| 1–2 times/week | 19 | 47.5% | 21 | 52.5% | ||||
| 3 times/week | 5 | 25.0% | 15 | 75.0% | ||||
| >3 times/week | 108 | 66.3% | 55 | 33.7% | ||||
| Pets in house | 0.370 | 0.193 | 0.707 | 0.002 | ||||
| Yes | 15 | 30.0% | 35 | 70.0% | ||||
| No | 153 | 53.7% | 132 | 46.3% | ||||
| Vit D intake | 0.707 | 0.460 | 1.086 | 0.113 | ||||
| Yes | 77 | 45.8% | 91 | 54.2% | ||||
| No | 91 | 54.5% | 76 | 45.5% | ||||
| Respiratory infection admissions > 2 times/year | 0.820 | 0.406 | 1.655 | 0.579 | ||||
| Yes | 16 | 45.7% | 19 | 54.3% | ||||
| No | 152 | 50.7% | 148 | 49.3% | ||||
| Gastroenteritis admissions > 2 times/year | 0.107 | 0.037 | 0.311 | <0.001 | ||||
| Yes | 4 | 11.4% | 31 | 88.6% | ||||
| No | 164 | 54.7% | 136 | 45.3% | ||||
| Bed share | 0.877 | 0.500 | 1.540 | 0.649 | ||||
| Yes | 28 | 47.5% | 31 | 52.5% | ||||
| No | 140 | 50.7% | 136 | 49.3% | ||||
| Diseases during pregnancy | 1.202 | 0.698 | 2.069 | 0.506 | ||||
| Yes | 35 | 53.8% | 30 | 46.2% | ||||
| No | 132 | 49.3% | 136 | 50.7% | ||||
| Diagnosis | |||||||
|---|---|---|---|---|---|---|---|
| CD | UC | Normal | p | ||||
| n | % | n | % | n | % | ||
| Age | <0.001 * | ||||||
| <3 years | 1 | 4.8% | 0 | 0.0% | 20 | 95.2% | |
| 3–6 years | 0 | 0.0% | 2 | 5.9% | 32 | 94.1% | |
| 10 years | 19 | 20.7% | 16 | 17.4% | 57 | 62.0% | |
| >10 years | 73 | 38.8% | 56 | 29.8% | 59 | 31.4% | |
| Fruit intake | <0.001 * | ||||||
| Yes | 19 | 17.6% | 18 | 16.7% | 71 | 65.7% | |
| No | 74 | 32.6% | 56 | 24.7% | 97 | 42.7% | |
| Exposure to antibiotics | 0.001 * | ||||||
| None | 56 | 35.2% | 42 | 26.4% | 61 | 38.4% | |
| Monthly | 8 | 23.5% | 4 | 11.8% | 22 | 64.7% | |
| Yearly | 29 | 20.4% | 28 | 19.7% | 85 | 59.9% | |
| Appendectomy | <0.001 ** | ||||||
| Yes | 10 | 71.4% | 4 | 28.6% | 0 | 0.0% | |
| No | 83 | 25.9% | 70 | 21.8% | 168 | 52.3% | |
| Family stress, anxiety | <0.001 * | ||||||
| Yes | 39 | 39.8% | 32 | 32.7% | 27 | 27.6% | |
| No | 54 | 22.8% | 42 | 17.7% | 141 | 59.5% | |
| Sleep disturbance | <0.001 * | ||||||
| Yes | 25 | 44.6% | 18 | 32.1% | 13 | 23.2% | |
| No | 68 | 24.4% | 56 | 20.1% | 155 | 55.6% | |
| Physical activity | <0.001 * | ||||||
| None | 38 | 33.9% | 38 | 33.9% | 36 | 32.1% | |
| 1–2 times/week | 11 | 27.5% | 10 | 25.0% | 19 | 47.5% | |
| 3 times/week | 8 | 40.0% | 7 | 35.0% | 5 | 25.0% | |
| >3 times/week | 36 | 22.1% | 19 | 11.7% | 108 | 66.3% | |
| Pets in house | 0.001 * | ||||||
| Yes | 15 | 30.0% | 20 | 40.0% | 15 | 30.0% | |
| No | 78 | 27.4% | 54 | 18.9% | 153 | 53.7% | |
| Type of delivery | 0.036 * | ||||||
| Vaginal | 65 | 29.5% | 55 | 25.0% | 100 | 45.5% | |
| Caesarean | 26 | 23.0% | 19 | 16.8% | 68 | 60.2% | |
| Gastroenteritis admissions > times/year | <0.001 * | ||||||
| Yes | 16 | 45.7% | 15 | 42.9% | 4 | 11.4% | |
| No | 77 | 25.7% | 59 | 19.7% | 164 | 54.7% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasosah, M.; Alhashmi, W.; Abualsaud, R.; Alamoudi, A.; Aljawad, A.; Tunkar, M.; Felemban, N.; Basalim, A.; Khan, M.; Alanazi, A.; et al. Environmental Risk Factors for Childhood Inflammatory Bowel Diseases: A Multicenter Case-Control Study. Children 2022, 9, 438. https://doi.org/10.3390/children9030438
Hasosah M, Alhashmi W, Abualsaud R, Alamoudi A, Aljawad A, Tunkar M, Felemban N, Basalim A, Khan M, Alanazi A, et al. Environmental Risk Factors for Childhood Inflammatory Bowel Diseases: A Multicenter Case-Control Study. Children. 2022; 9(3):438. https://doi.org/10.3390/children9030438
Chicago/Turabian StyleHasosah, Mohammed, Wafaa Alhashmi, Renad Abualsaud, Anas Alamoudi, Afnan Aljawad, Mariam Tunkar, Nooran Felemban, Ahmed Basalim, Muhammad Khan, Aziz Alanazi, and et al. 2022. "Environmental Risk Factors for Childhood Inflammatory Bowel Diseases: A Multicenter Case-Control Study" Children 9, no. 3: 438. https://doi.org/10.3390/children9030438