Feasibility and Safety of Percutaneous Cardiac Interventions for Congenital and Acquired Heart Defects in Infants ≤1000 g
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Data Analysis
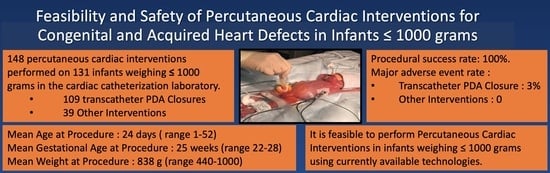
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Center for Health Statistics. Final Natality Data. Available online: www.marchofdimes.org/peristats (accessed on 10 August 2021).
- Gupta, S.; Adhisivam, B.; Bhat, B.V.; Plakkal, N.; Amala, R. Short Term Outcome and Predictors of Mortality Among Very Low Birth Weight Infants—A Descriptive Study. Indian J. Pediatr. 2021, 88, 351–357. [Google Scholar] [CrossRef]
- Avsar, M.K.; Demir, T.; Celiksular, C.; Zeybek, C. Bedside PDA ligation in premature infants less than 28 weeks and 1000 grams. J. Cardiothorac. Surg. 2016, 11, 146. [Google Scholar] [CrossRef] [Green Version]
- Zahn, E.M.; Peck, D.; Phillips, A.; Nevin, P.; Basaker, K.; Simmons, C.; McRae, M.E.; Early, T.; Garg, R. Transcatheter Closure of Patent Ductus Arteriosus in Extremely Premature Newborns: Early Results and Midterm Follow-Up. JACC Cardiovasc. Interv. 2016, 9, 2429–2437. [Google Scholar] [CrossRef]
- Backes, C.H.; Cua, C.; Kreutzer, J.; Armsby, L.; El-Said, H.; Moore, J.W.; Gauvreau, K.; Bergersen, L.; Holzer, R.J. Low weight as an independent risk factor for adverse events during cardiac catheterization of infants. Catheter Cardiovasc. Interv. 2013, 82, 786–794. [Google Scholar] [CrossRef]
- Backes, C.H.; Cheatham, S.L.; Deyo, G.M.; Leopold, S.; Ball, M.K.; Smith, C.V.; Garg, V.; Holzer, R.J.; Cheatham, J.P.; Berman, D.P. Percutaneous Patent Ductus Arteriosus (PDA) Closure in Very Preterm Infants: Feasibility and Complications. J. Am. Heart Assoc. 2016, 5, e002923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahn, E.M.; Nevin, P.; Simmons, C.; Garg, R. A novel technique for transcatheter patent ductus arteriosus closure in extremely preterm infants using commercially available technology. Catheter Cardiovasc. Interv. 2015, 85, 240–248. [Google Scholar] [CrossRef]
- Philip, R.; Waller, B.R., 3rd; Agrawal, V.; Wright, D.; Arevalo, A.; Zurakowski, D.; Sathanandam, S. Morphologic characterization of the patent ductus arteriosus in the premature infant and the choice of transcatheter occlusion device. Catheter Cardiovasc. Interv. 2016, 87, 310–317. [Google Scholar] [CrossRef]
- Sathanandam, S.; Justino, H.; Waller, B.R., 3rd; Radtke, W.; Qureshi, A.M. Initial clinical experience with the Medtronic Micro Vascular Plug™ in transcatheter occlusion of PDAs in extremely premature infants. Catheter Cardiovasc. Interv. 2017, 89, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Sathanandam, S.; Balduf, K.; Chilakala, S.; Washington, K.; Allen, K.; Knott-Craig, C.; Rush Waller, B.; Philip, R. Role of Transcatheter patent ductus arteriosus closure in extremely low birth weight infants. Catheter Cardiovasc. Interv. 2019, 93, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sathanandam, S.K.; Gutfinger, D.; O’Brien, L.; Forbes, T.J.; Gillespie, M.J.; Berman, D.P.; Armstrong, A.K.; Shahanavaz, S.; Jones, T.K.; Morray, B.H.; et al. Amplatzer Piccolo Occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients ≥700 grams. Catheter Cardiovasc. Interv. 2020, 96, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Sutton, N.; Lock, J.E.; Geggel, R.L. Cardiac catheterization in infants weighing less than 1500 grams. Catheter Cardiovasc. Interv. 2006, 68, 948–956. [Google Scholar] [CrossRef]
- Zahn, E.M.; Abbott, E.; Tailor, N.; Sathanandam, S.; Armer, D. Preliminary testing and evaluation of the renata minima stent, an infant stent capable of achieving adult dimensions. Catheter Cardiovasc. Interv. 2021, 98, 117–127. [Google Scholar] [CrossRef]
- Philip, R.; Waller, B.R.; Chilakala, S.; Graham, B.; Stecchi, N.; Apalodimas, L.; Cunningham, J.; Washington, K.; Sathanandam, S. Hemodynamic and clinical consequences of early versus delayed closure of patent ductus arteriosus in extremely low birth weight infants. J. Perinatol. 2021, 41, 100–108. [Google Scholar] [CrossRef]
- Bischoff, A.R.; Jasani, B.; Sathanandam, S.K.; Backes, C.; Weisz, D.E.; McNamara, P.J. Percutaneous Closure of Patent Ductus Arteriosus in Infants 1.5 kg or Less: A Meta-Analysis. J. Pediatr. 2021, 230, 84–92.e14. [Google Scholar] [CrossRef] [PubMed]
- Sathanandam, S.; Agrawal, H.; Chilakala, S.; Johnson, J.; Allen, K.; Knott-Craig, C.; Rush Waller, B.; Philip, R. Can transcatheter PDA closure be performed in neonates ≤1000 grams? The Memphis experience. Congenit Heart Dis. 2019, 14, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.N.; Sathanandam, S.; Naik, R.; Philip, R. Echocardiographic guidance for transcatheter patent ductus arteriosus closure in extremely low birth weight infants. Congenit Heart Dis. 2019, 14, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Paudel, G.; Johnson, J.N.; Philip, R.; Tailor, N.; Fahnhorst, S.; Briceno-Medina, M.; Stecchi, N.; Waller, B.R.; Sathanandam, S. Echocardiographic versus Angiographic Measurement of the Patent Ductus Arteriosus in Extremely Low Birth Weight Infants and the Utility of Echo Guidance for Transcatheter Closure. J. Am. Soc. Echocardiogr. 2021, 15, 74–78. [Google Scholar]
- Paudel, G.; Joshi, V. Echocardiography of the patent ductus arteriosus in premature infant. Congenit Heart Dis. 2019, 14, 42–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willis, A.; Pereiras, L.; Head, T.; Dupuis, G.; Sessums, J.; Corder, G.; Graves, K.; Tipton, J.; Sathanandam, S. Transport of extremely low birth weight neonates for persistent ductus arteriosus closure in the catheterization lab. Congenit Heart Dis. 2019, 14, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philip, R.; Tailor, N.; Johnson, J.N.; Apalodimas, L.; Cunningham, J.; Hoy, J.; Waller, B.R., III; Sathanandam, S. Single-Center Experience of 100 Consecutive Percutaneous Patent Ductus Arteriosus Closures in Infants ≤1000 Grams. Circ. Cardiovasc. Interv. 2021, 14, e010600. [Google Scholar] [CrossRef]
- Apalodimas, L.; Waller, B.R., III; Philip, R.; Crawford, J.; Cunningham, J.; Sathanandam, S. A comprehensive program for preterm infants with patent ductus arteriosus. Congenit Heart Dis. 2019, 14, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Sathanandam, S.K.; Kumar, T.K.; Hoskoppal, D.; Haddad, L.M.; Subramanian, S.; Sullivan, R.D.; Zurakowski, D.; Knott-Craig, C.; Waller, B.R., 3rd. Feasibility and Safety of Unzipping Small Diameter Stents in the Blood Vessels of Piglets. JACC Cardiovasc. Interv. 2016, 9, 1138–1149. [Google Scholar] [CrossRef]
- Sathanandam, S.K.; Haddad, L.M.; Subramanian, S.; Wright, D.; Philip, R.; Waller, B. R Unzipping of small diameter stents: An in vitro study. Catheter Cardiovasc. Interv. 2015, 85, 249–258. [Google Scholar] [CrossRef]
- Brumbaugh, J.E.; Hansen, N.I.; Bell, E.F.; Sridhar, A.; Carlo, W.A.; Hintz, S.R.; Vohr, B.R.; Colaizy, T.T.; Duncan, A.F.; Wyckoff, M.H.; et al. National Institute of Child Health and Human Development Neonatal Research Network. Outcomes of Extremely Preterm Infants With Birth Weight Less Than 400 g. JAMA Pediatr. 2019, 173, 434–445. [Google Scholar] [CrossRef]
- Alexander, J.; Yohannan, T.; Abutineh, I.; Agrawal, V.; Lloyd, H.; Zurakowski, D.; Waller, B.R., 3rd; Sathanandam, S. Ultrasound-guided femoral arterial access in pediatric cardiac catheterizations: A prospective evaluation of the prevalence, risk factors, and mechanism for acute loss of arterial pulse. Catheter Cardiovasc. Interv. 2016, 88, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Tadphale, S.D.; Zurakowski, D.; Bird, L.E.; Yohannan, T.M.; Agrawal, V.K.; Lloyd, H.G.; Allen, K.J.; Waller, B.R., 3rd; Hall, A.M.; Sathanandam, S.K. Construction of Femoral Vessel Nomograms for Planning Cardiac Interventional Procedures in Children 0-4 Years Old. Pediatr Cardiol. 2020, 41, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Tadphale, S.; Yohannan, T.; Kauffmann, T.; Maller, V.; Agrawal, V.; Lloyd, H.; Waller, B.R.; Sathanandam, S. Accessing Femoral Arteries Less than 3 mm in Diameter is Associated with Increased Incidence of Loss of Pulse Following Cardiac Catheterization in Infants. Pediatr Cardiol. 2020, 41, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Sathanandam, S.; Gutfinger, D.; Morray, B.; Berman, D.; Gillespie, M.; Forbes, T.; Johnson, J.N.; Garg, R.; Malekzadeh-Milani, S.; Fraisse, A.; et al. Consensus Guidelines for the Prevention and Management of Periprocedural Complications of Transcatheter Patent Ductus Arteriosus Closure with the Amplatzer Piccolo Occluder in Extremely Low Birth Weight Infants. Pediatr. Cardiol. 2021, 42, 1258–1274. [Google Scholar] [CrossRef]
- Khan, A.H.; Hoskoppal, D.; Kumar, T.K.S.; Bird, L.; Allen, K.; Lloyd, H.; Knott-Craig, C.J.; Waller, B.R.; Sathanandam, S. Utility of the Medtronic microvascular plug™ as a transcatheter implantable and explantable pulmonary artery flow restrictor in a swine model. Catheter Cardiovasc. Interv. 2019, 93, 1320–1328. [Google Scholar] [CrossRef]
- Kiene, A.M.; Waller, B.R., 3rd; Craig, C.K.; Sathanandam, S. Percutaneous Stage 1 Palliation for Hypoplastic Left Heart Syndrome. Ann. Thorac Surg. 2021, 16. [Google Scholar] [CrossRef]




| Procedure Type | n |
|---|---|
| 1. Transcatheter PDA Closure | 109 |
| 2. Pulmonary Valvuloplasty | 9 |
| 3. Foreign Body Retrieval | 8 |
| 4. Pericardiocentesis | 6 |
| 5. Coarctation of the aorta stent | 6 |
| 6. Ductal Stent | 2 |
| 7. Pulmonary valve perforation for pulmonary atresia | 2 |
| 8. Coiling of artery to pulmonary sequestration | 2 |
| 9. Balloon atrial septostomy | 1 |
| 10. Radiofrequency perforation for HLHS with intact atrial septum with inter-atrial stent placement | 1 |
| 11. Pulmonary artery balloon angioplasty | 1 |
| 12. Pulmonary artery flow reducer | 1 |
| Non-TCPC Interventions (n = 39) | |
|---|---|
| Procedure Weight (g) | |
| Mean ± SD | 838 ± 169 |
| Median (Range) | 900 (440–1000) |
| Procedure Age (days) | |
| Mean ± SD | 24 ± 12 |
| Median (Range) | 28 (1–52) |
| Gestational Age (weeks) | |
| Mean ± SD | 25 ± 1 |
| Median (Range) | 25 (22–28) |
| Birth Weight (g) | |
| Mean ± SD | 750 ± 172 |
| Median (Range) | 700 (400–980) |
| Sex, Male | 54% (21) |
| Procedure Type | n | Major AE n (%) | Minor AE n (%) | Survival at Latest F/U (Median = 3 Years) |
|---|---|---|---|---|
| 1. Transcatheter PDA Closure (TCPC) *^‡¶œ | 109 | 3 | 3 | 99 |
| 2. Pulmonary Valvuloplasty *∂€ | 9 | 0 | 0 | 6 |
| 3. Foreign Body Retrieval ^ | 8 | 0 | 0 | 8 |
| 4. Pericardiocentesis ‡ | 6 | 0 | 0 | 5 |
| 5. Coarctation of the aorta stent | 6 | 0 | 1 | 3 |
| 6. Ductal Stent ∂† | 2 | 0 | 0 | 1 |
| 7. Pulmonary valve perforation for pulmonary atresia €∂ | 2 | 0 | 0 | 1 |
| 8. Coiling of artery to pulmonary sequestration œ | 2 | 0 | 0 | 2 |
| 9. Balloon atrial septostomy | 1 | 0 | 0 | 0 |
| 10. Radiofrequency perforation for HLHS with intact atrial septum with inter-atrial stent placement † | 1 | 0 | 0 | 0 |
| 11. Pulmonary artery balloon angioplasty | 1 | 0 | 0 | 0 |
| 12. Pulmonary artery flow reducer ∂ | 1 | 0 | 0 | 1 |
| Overall Total | 148 | 3 (2%) | 4 (2.7%) | 87.8% (115/131) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philip, R.; Towbin, J.; Tailor, N.; Joshi, V.; Johnson, J.N.; Naik, R.; Waller, B.R., III; Sathanandam, S. Feasibility and Safety of Percutaneous Cardiac Interventions for Congenital and Acquired Heart Defects in Infants ≤1000 g. Children 2021, 8, 826. https://doi.org/10.3390/children8090826
Philip R, Towbin J, Tailor N, Joshi V, Johnson JN, Naik R, Waller BR III, Sathanandam S. Feasibility and Safety of Percutaneous Cardiac Interventions for Congenital and Acquired Heart Defects in Infants ≤1000 g. Children. 2021; 8(9):826. https://doi.org/10.3390/children8090826
Chicago/Turabian StylePhilip, Ranjit, Jeffrey Towbin, Neil Tailor, Vijaya Joshi, Jason N. Johnson, Ronak Naik, B. Rush Waller, III, and Shyam Sathanandam. 2021. "Feasibility and Safety of Percutaneous Cardiac Interventions for Congenital and Acquired Heart Defects in Infants ≤1000 g" Children 8, no. 9: 826. https://doi.org/10.3390/children8090826