Waist Circumference Is Not Associated with Impaired Fasting Blood Glucose in a Sample of Mexican Children and Teenagers: Results from a State Screening Program
Abstract
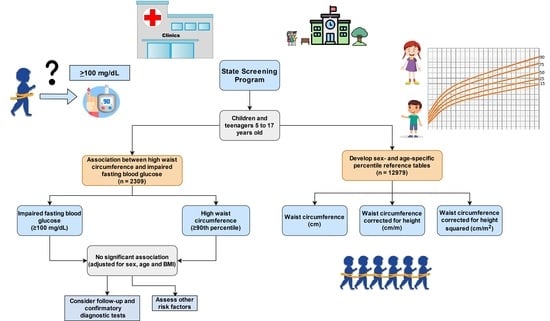
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Assessment
2.3. Blood Glucose Assessment
2.4. Statistical Analysis
3. Results
3.1. Anthropometric Comparisons by Age
3.2. Waist Circumference Percentiles
3.3. Subsample Analysis and Fasting Blood Glucose
3.4. Waist Circumference and Impaired Fasting Blood Glucose Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Maung-U, K.; Jagadeesh, G. Prevalence and Prevention of Cardiovascular Disease and Diabetes Mellitus. Pharmacol. Res. 2016, 113, 600–609. [Google Scholar] [CrossRef] [PubMed]
- The GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch-Blüher, S.; Wiegand, S. Risk Factors and Implications of Childhood Obesity. Curr. Obes. Rep. 2018, 7, 254–259. [Google Scholar] [CrossRef]
- Xu, H.; Verre, M.C. Type 2 Diabetes Mellitus in Children. Am. Fam. Physician 2018, 98, 590–594. [Google Scholar]
- Pastore, I.; Bolla, A.M.; Montefusco, L.; Lunati, M.E.; Rossi, A.; Assi, E.; Zuccotti, G.V.; Fiorina, P. The Impact of Diabetes Mellitus on Cardiovascular Risk Onset in Children and Adolescents. Int. J. Mol. Sci. 2020, 21, 4928. [Google Scholar] [CrossRef] [PubMed]
- Shamah-Levy, T.; Vielma-Orozco, E.; Heredia-Hernández, O.; Romero-Martínez, M.; Mojica-Cuevas, J.; Cuevas-Nasu, L.; Santaella-Castell, J.A.; Rivera-Dommarco, J. Encuesta Nacional de Salud y Nutrición 2018–19. Resultados Nacionales; Instituto Nacional de Salud Pública: Cuernavaca, México, 2020. [Google Scholar]
- Bello-Chavolla, O.Y.; Rojas-Martinez, R.; Aguilar-Salinas, C.A.; Hernández-Avila, M. Epidemiology of Diabetes Mellitus in Mexico. Nutr. Rev. 2017, 75 (Suppl. 1), 4–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Yoon, K.H. Epidemic Obesity in Children and Adolescents: Risk Factors and Prevention. Front. Med. 2018, 12, 658–666. [Google Scholar] [CrossRef]
- Martinez, L.C.; Sherling, D.; Holley, A. The Screening and Prevention of Diabetes Mellitus. Prim. Care 2019, 46, 41–52. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef]
- Barry, E.; Roberts, S.; Oke, J.; Vijayaraghavan, S.; Normansell, R.; Greenhalgh, T. Efficacy and Effectiveness of Screen and Treat Policies in Prevention of Type 2 Diabetes: Systematic Review and Meta-Analysis of Screening Tests and Interventions. BMJ 2017, 356, i6538. [Google Scholar] [CrossRef] [Green Version]
- Molina-Luque, R.; Ulloa, N.; Gleisner, A.; Zilic, M.; Romero-Saldaña, M.; Molina-Recio, G. An Approach to Early Detection of Metabolic Syndrome through Non-Invasive Methods in Obese Children. Children 2020, 7, 304. [Google Scholar] [CrossRef] [PubMed]
- Rodea-Montero, E.R.; Evia-Viscarra, M.L.; Apolinar-Jiménez, E. Waist-to-Height Ratio Is a Better Anthropometric Index than Waist Circumference and BMI in Predicting Metabolic Syndrome among Obese Mexican Adolescents. Int. J. Endocrinol. 2014, 2014, 195407. [Google Scholar] [CrossRef]
- Aguilar-Morales, I.; Colin-Ramirez, E.; Rivera-Mancía, S.; Vallejo, M.; Vázquez-Antona, C. Performance of Waist-to-Height Ratio, Waist Circumference, and Body Mass Index in Discriminating Cardio-Metabolic Risk Factors in a Sample of School-Aged Mexican Children. Nutrients 2018, 10, 1850. [Google Scholar] [CrossRef] [Green Version]
- Alves Junior, C.A.S.; Mocellin, M.C.; Gonçalves, E.C.A.; Silva, D.A.S.; Trindade, E.B.S.M. Anthropometric Indicators as Body Fat Discriminators in Children and Adolescents: A Systematic Review and Meta-Analysis. Adv. Nutr. 2017, 8, 718–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skinner, A.C.; Perrin, E.M.; Moss, L.A.; Skelton, J.A. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N. Engl. J. Med. 2015, 373, 1307–1317. [Google Scholar] [CrossRef]
- Iacobini, C.; Pugliese, G.; Blasetti Fantauzzi, C.; Federici, M.; Menini, S. Metabolically Healthy versus Metabolically Unhealthy Obesity. Metabolism 2019, 92, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N. Causes, Consequences, and Treatment of Metabolically Unhealthy Fat Distribution. Lancet Diabetes Endocrinol. 2020, 8, 616–627. [Google Scholar] [CrossRef]
- Samouda, H.; De Beaufort, C.; Stranges, S.; Van Nieuwenhuyse, J.P.; Dooms, G.; Keunen, O.; Leite, S.; Vaillant, M.; Lair, M.L.; Dadoun, F. Subtraction of Subcutaneous Fat to Improve the Prediction of Visceral Adiposity: Exploring a New Anthropometric Track in Overweight and Obese Youth. Pediatr. Diabetes 2017, 18, 399–404. [Google Scholar] [CrossRef]
- Brambilla, P.; Bedogni, G.; Moreno, L.A.; Goran, M.I.; Gutin, B.; Fox, K.R.; Peters, D.M.; Barbeau, P.; De Simone, M.; Pietrobelli, A. Crossvalidation of Anthropometry against Magnetic Resonance Imaging for the Assessment of Visceral and Subcutaneous Adipose Tissue in Children. Int. J. Obes. 2006, 30, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Bassali, R.; Waller, J.L.; Gower, B.; Allison, J.; Davis, C.L. Utility of Waist Circumference Percentile for Risk Evaluation in Obese Children. Int. J. Pediatr. Obes. 2010, 5, 97–101. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.G.; Guedes, D.P. Performance of Anthropometric Indicators as Predictors of Metabolic Syndrome in Brazilian Adolescents. BMC Pediatr. 2018, 18, 33. [Google Scholar] [CrossRef] [Green Version]
- López-González, D.; Miranda-Lora, A.; Klünder-Klünder, M.; Queipo-García, G.; Bustos-Esquivel, M.; Paez-Villa, M.; Villanueva-Ortega, E.; Chávez-Requena, I.; Laresgoiti-Servitje, E.; Garibay-Nieto, N. Diagnostic Performance of Waist Circumference Measurements for Predicting Cardiometabolic Risk in Mexican Children. Endocr. Pract. 2016, 22, 1170–1176. [Google Scholar] [CrossRef]
- Janssen, I.; Katzmarzyk, P.T.; Srinivasan, S.R.; Chen, W.; Malina, R.M.; Bouchard, C.; Berenson, G.S. Combined Influence of Body Mass Index and Waist Circumference on Coronary Artery Disease Risk Factors among Children and Adolescents. Pediatrics 2005, 115, 1623. [Google Scholar] [CrossRef]
- Kuba, V.M.; Leone, C.; Damiani, D. Is Waist-to-Height Ratio a Useful Indicator of Cardio-Metabolic Risk in 6-10-Year-Old Children? BMC Pediatr. 2013, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elizondo-Montemayor, L.; Serrano-González, M.; Ugalde-Casas, P.A.; Bustamante-Careaga, H.; Cuello-García, C. Waist-to-Height: Cutoff Matters in Predicting Metabolic Syndrome in Mexican Children. Metab. Syndr. Relat. Disord. 2011, 9, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.R.C.; Cardoso, P.C.; Frossard, T.N.S.V.; de Oliveira Ferreira, F.; Brener, S.; de Freitas Gomides, A.F.; Valente, M.A.S.; Velloso-Rodrigues, C. Waist Circumference as High Blood Pressure Predictor in School Age Children. Cien. Saude Colet. 2019, 24, 1885–1893. [Google Scholar] [CrossRef] [Green Version]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics 2011, 128, S213–S256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q.H. Novel Insights into the Pathogenesis and Management of the Metabolic Syndrome. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 189–230. [Google Scholar] [CrossRef]
- Xi, B.; Zong, X.; Kelishadi, R.; Litwin, M.; Hong, Y.M.; Poh, B.K.; Steffen, L.M.; Galcheva, S.V.; Herter-Aeberli, I.; Nawarycz, T.; et al. International Waist Circumference Percentile Cutoffs for Central Obesity in Children and Adolescents Aged 6 to 18 Years. J. Clin. Endocrinol. Metab. 2020, 105, E1569–E1583. [Google Scholar] [CrossRef]
- Kleber, M.; Sousa, G.; Papcke, S.; Reinehr, T. Risk Factors for Impaired Glucose Tolerance in Obese Children and Adolescents. World J. Diabetes 2010, 1, 129. [Google Scholar] [CrossRef] [PubMed]
- Morandi, A.; Miraglia Del Giudice, E.; Martino, F.; Martino, E.; Bozzola, M.; Maffeis, C. Anthropometric Indices Are Not Satisfactory Predictors of Metabolic Comorbidities in Obese Children and Adolescents. J. Pediatr. 2014, 165, 1178–1183.e2. [Google Scholar] [CrossRef] [PubMed]
- Marrodán Serrano, M.D.; Román, E.; Carmenate, M.; González-Montero de Espinosa, M.; Herráez, A.; Alfaro, E.L.; Lomaglio, D.B.; López-Ejeda, N.; Mesa, M.S.; Vázquez, V.; et al. Waist Circumference Percentiles for Hispanic-American Children and Comparison with Other International References. Am. J. Hum. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Messiah, S.E.; Arheart, K.L.; Lipshultz, S.E.; Miller, T.L. Ethnic Group Differences in Waist Circumference Percentiles among U.S. Children and Adolescents: Estimates from the 1999–2008 National Health and Nutrition Examination Surveys. Metab. Syndr. Relat. Disord. 2011, 9, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.R.; Redden, D.T.; Pietrobelli, A.; Allison, D.B. Waist Circumference Percentiles in Nationally Representative Samples of African-American, European-American, and Mexican-American Children and Adolescents. J. Pediatr. 2004, 145, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Klünder-Klünder, M.; Flores-Huerta, S. Waist Circumference Values According to Height Percentiles: A Proposal to Evaluate Abdominal Obesity in Mexican Children and Adolescents Between 6 and 16 Years of Age. Arch. Med. Res. 2011, 42, 515–522. [Google Scholar] [CrossRef]
- Kuriyan, R.; Thomas, T.; Lokesh, D.P.; Sheth, N.R.; Mahendra, A.; Joy, R.; Sumithra, S.; Bhat, S.; Kurpad, A.V. Waist Circumference and Waist for Height Percentiles in Urban South Indian Children Aged 3–16 Years. Indian Pediatr. 2011, 48, 765–771. [Google Scholar] [CrossRef]
- Gandhi, K. Approach to Hypoglycemia in Infants and Children. Transl. Pediatr. 2017, 6, 408–420. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. BMI-for-Age (5–19 Years). Available online: https://www.who.int/toolkits/growth-reference-data-for-5to19-years/indicators/bmi-for-age (accessed on 28 January 2021).
- De Onis, M.; Martínez-Costa, C.; Núñez, F.; Nguefack-Tsague, G.; Montal, A.; Brines, J. Association between WHO Cut-Offs for Childhood Overweight and Obesity and Cardiometabolic Risk. Public Health Nutr. 2013, 16, 625–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO STEPS Surveillance Manual. The WHO STEPwise Approach to Noncommunicable Disease Risk Factor Surveillance; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Volker, M.A. Reporting Effect Size Estimates in School Psychology Research. Psychol. Sch. 2006, 43, 653–672. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the p Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [Green Version]
- Berber, A.; Gómez-Santos, R.; Fanghänel, G.; Sánchez-Reyes, L. Anthropometric Indexes in the Prediction of Type 2 Diabetes Mellitus, Hypertension and Dyslipidaemia in a Mexican Population. Int. J. Obes. 2001, 25, 1794–1799. [Google Scholar] [CrossRef] [Green Version]
- Harada, Y.; Harada, K.; Chin, P. Comparing Self Monitoring Blood Glucose Devices and Laboratory Tests: Over 25 Years Experience. Cureus 2019, 11, e6268. [Google Scholar] [CrossRef] [Green Version]
- Savic Hitt, T.A.; Katz, L.E.L. Pediatric Type 2 Diabetes: Not a Mini Version of Adult Type 2 Diabetes. Endocrinol. Metab. Clin. N. Am. 2020, 49, 679–693. [Google Scholar] [CrossRef]
- Harrington, D.M.; Staiano, A.E.; Broyles, S.T.; Gupta, A.K.; Katzmarzyk, P.T. Waist Circumference Measurement Site Does Not Affect Relationships with Visceral Adiposity and Cardiometabolic Risk Factors in Children. Pediatr. Obes. 2013, 8, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andes, L.J.; Cheng, Y.J.; Rolka, D.B.; Gregg, E.W.; Imperatore, G. Prevalence of Prediabetes among Adolescents and Young Adults in the United States, 2005-2016. JAMA Pediatr. 2020, 174. [Google Scholar] [CrossRef] [PubMed]
- Benyi, E.; Sävendahl, L. The Physiology of Childhood Growth: Hormonal Regulation. Horm. Res. Paediatr. 2017, 88, 6–14. [Google Scholar] [CrossRef]
- Arslanian, S.; Bacha, F.; Grey, M.; Marcus, M.D.; White, N.H.; Zeitler, P. Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care 2018, 41, dci180052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslanian, S.; Kim, J.Y.; Nasr, A.; Bacha, F.; Tfayli, H.; Lee, S.J.; Toledo, F.G.S. Insulin Sensitivity across the Lifespan from Obese Adolescents to Obese Adults with Impaired Glucose Tolerance: Who Is Worse Off? Pediatr. Diabetes 2018, 19, 205–211. [Google Scholar] [CrossRef] [PubMed]
- van der Aa, M.P.; Fazeli Farsani, S.; Knibbe, C.A.J.; de Boer, A.; van der Vorst, M.M.J. Population-Based Studies on the Epidemiology of Insulin Resistance in Children. J. Diabetes Res. 2015, 2015, 362375. [Google Scholar] [CrossRef] [Green Version]
- Ekelund, U.; Luan, J.; Sherar, L.B.; Esliger, D.W.; Griew, P.; Cooper, A. Moderate to Vigorous Physical Activity and Sedentary Time and Cardiometabolic Risk Factors in Children and Adolescents. JAMA 2012, 307, 704–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loprinzi, P.D.; Cardinal, B.J.; Lee, H.; Tudor-Locke, C. Markers of Adiposity among Children and Adolescents: Implications of the Isotemporal Substitution Paradigm with Sedentary Behavior and Physical Activity Patterns. J. Diabetes Metab. Disord. 2015, 14, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalene, K.E.; Anderssen, S.A.; Andersen, L.B.; Steene-Johannessen, J.; Ekelund, U.; Hansen, B.H.; Kolle, E. Cross-Sectional and Prospective Associations between Physical Activity, Body Mass Index and Waist Circumference in Children and Adolescents. Obes. Sci. Pract. 2017, 3, 249–257. [Google Scholar] [CrossRef]
- Ashwell, M.; Hsieh, S.D. Six Reasons Why the Waist-to-Height Ratio Is a Rapid and Effective Global Indicator for Health Risks of Obesity and How Its Use Could Simplify the International Public Health Message on Obesity. Int. J. Food Sci. Nutr. 2005, 56, 303–307. [Google Scholar] [CrossRef]
- Chiarelli, F.; Marcovecchio, M.L. Insulin Resistance and Obesity in Childhood. Eur. J. Endocrinol. 2008, 159 (Suppl. 1), 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelsey, M.M.; Severn, C.; Hilkin, A.M.; Pyle, L.; Nadeau, K.J.; Zeitler, P.S. Puberty Is Associated with a Rising Hemoglobin A1c, Even in Youth with Normal Weight. J. Pediatr. 2020. [Google Scholar] [CrossRef]
- Berman, L.J.; Weigensberg, M.J.; Spruijt-Metz, D. Physical Activity Is Related to Insulin Sensitivity in Children and Adolescents, Independent of Adiposity: A Review of the Literature. Diabetes Metab. Res. Rev. 2012, 28, 395–408. [Google Scholar] [CrossRef]
- King, F.; Ahn, D.; Hsiao, V.; Porco, T.; Klonoff, D.C. A Review of Blood Glucose Monitor Accuracy. Diabetes Technol. Ther. 2018, 20, 843–856. [Google Scholar] [CrossRef]
- Pleus, S.; Baumstark, A.; Jendrike, N.; Mende, J.; Link, M.; Zschornack, E.; Haug, C.; Freckmann, G. System Accuracy Evaluation of 18 CE-Marked Current-Generation Blood Glucose Monitoring Systems Based on En ISO 15197:2015. BMJ Open Diabetes Res. Care 2020, 8, e001067. [Google Scholar] [CrossRef] [Green Version]



| Total (n = 12979) | Girls (n = 6998) | Boys (n = 5981) | p-Value § | φ | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Underweight | 320 | 2.5 | 150 | 2.1 | 170 | 2.8 | 0.012 | 0.022 |
| Normal | 7570 | 58.3 | 4192 | 59.9 | 3378 | 56.5 | <0.001 | 0.035 |
| Overweight | 2796 | 21.5 | 1604 | 22.9 | 1192 | 19.9 | <0.001 | 0.036 |
| Obesity | 2293 | 17.7 | 1052 | 15.0 | 1241 | 20.7 | <0.001 | 0.075 |
| Age Group (Years) | p-Trend § | ||||||
|---|---|---|---|---|---|---|---|
| 5 to 6.9 | 7 to 8.9 | 9 to 10.9 | 11 to 12.9 | 13 to 14.9 | 15 to 17.9 | ||
| Girls (n = 1271) | |||||||
| n | 100 | 174 | 200 | 216 | 232 | 349 | |
| FBG (mg/dL) † | 92 a,b,c (85–97) | 93 a,c (86–98) | 95 a (89–102) | 95 a,c (85–101) | 92 b,c (84–98) | 90 b (84–96) | <0.001 |
| IFG ‡ | 18 a,b (18.0) | 40 a,b (23.0) | 57 b (28.5) | 65 b (30.1) | 50 a,b (21.6) | 49 a (14.0) | <0.001 |
| Boys (n = 1038) | |||||||
| n | 87 | 137 | 178 | 190 | 205 | 241 | |
| FBG (mg/dL) | 94 a,b (82–100) | 96 a (88–102) | 95 a (90–102) | 96 a (87–103) | 94 a,b (86–99) | 92 b (85–99) | 0.001 |
| IFG | 24 a,b (27.6) | 43 a,b (31.4) | 50 a,b (28.1) | 69 b (36.3) | 47 a,b (22.9) | 53 a (22.0) | 0.014 |
| Age Group (Years) | Cut Point † | Participants above the Cut Point (n) | Model 1 | Model 2 | ||
|---|---|---|---|---|---|---|
| OR (95% CI) ‡ | p-Value | OR (95% CI) | p-Value | |||
| WC (cm) | ||||||
| 5 to 6.9 | 66.3 | 10 | 2.14 (0.50–9.24) | 0.31 | 1.87 (0.26–13.3) | 0.53 |
| 7 to 8.9 | 72.9 | 18 | 3.10 (1.13–8.49) | 0.03 | 1.74 (0.39–7.72) | 0.47 |
| 9 to 10.9 | 81.0 | 21 | 2.05 (0.81–5.16) | 0.13 | 1.42 (0.45–4.53) | 0.55 |
| 11 to 12.9 | 87.0 | 22 | 0.86 (0.32–2.30) | 0.76 | 0.52 (0.15–1.83) | 0.31 |
| 13 to 14.9 | 89.0 | 25 | 1.48 (0.58–3.78) | 0.41 | 1.25 (0.34–4.62) | 0.74 |
| 15 to 17.9 | 90.0 | 35 | 1.62 (0.67–3.94) | 0.29 | 0.75 (0.23–2.46) | 0.64 |
| WC/HT (cm/m) | ||||||
| 5 to 6.9 | 55.71 | 10 | 2.14 (0.50–9.24) | 0.31 | 1.84 (0.36–9.49) | 0.47 |
| 7 to 8.9 | 54.69 | 18 | 3.10 (1.13–8.49) | 0.03 | 1.59 (0.32–7.93) | 0.57 |
| 9 to 10.9 | 57.66 | 20 | 1.78 (0.69–4.62) | 0.24 | 1.15 (0.36–3.69) | 0.82 |
| 11 to 12.9 | 56.13 | 22 | 0.86 (0.32–2.30) | 0.76 | 0.56 (0.16–1.89) | 0.35 |
| 13 to 14.9 | 55.15 | 24 | 2.44 (1.00–5.98) | 0.05 | 3.14 (0.92–10.7) | 0.07 |
| 15 to 17.9 | 56.55 | 35 | 1.62 (0.67–3.94) | 0.29 | 0.79 (0.25–2.49) | 0.68 |
| WC/HT2 (cm/m2) | ||||||
| 5 to 6.9 | 48.63 | 10 | 0.48 (0.06–4.02) | 0.50 | 0.44 (0.05–3.81) | 0.46 |
| 7 to 8.9 | 43.59 | 18 | 2.37 (0.85–6.60) | 0.10 | 1.30 (0.37–4.60) | 0.68 |
| 9 to 10.9 | 41.25 | 20 | 1.40 (0.53–3.71) | 0.50 | 0.87 (0.28–2.70) | 0.82 |
| 11 to 12.9 | 37.11 | 22 | 0.66 (0.23–1.86) | 0.43 | 0.49 (0.15–1.56) | 0.23 |
| 13 to 14.9 | 35.14 | 24 | 1.58 (0.62–4.05) | 0.34 | 1.50 (0.51–4.46) | 0.46 |
| 15 to 17.9 | 36.05 | 35 | 2.37 (1.04–5.42) | 0.04 | 1.61 (0.58–4.47) | 0.36 |
| Age Group (Years) | Cut Point † | Participants above the Cut Point (n) | Model 1 | Model 2 | ||
|---|---|---|---|---|---|---|
| OR (95% CI) ‡ | p-Value | OR (95% CI) | p-Value | |||
| WC (cm) | ||||||
| 5 to 6.9 | 68.0 | 10 | 1.14 (0.27–4.84) | 0.86 | 1.29 (0.24–6.88) | 0.77 |
| 7 to 8.9 | 75.0 | 14 | 0.57 (0.15–2.14) | 0.40 | 0.75 (0.14–4.17) | 0.74 |
| 9 to 10.9 | 87.0 | 18 | 1.32 (0.47–3.73) | 0.60 | 1.25 (0.35–4.49) | 0.73 |
| 11 to 12.9 | 91.2 | 19 | 1.31 (0.50–3.44) | 0.58 | 1.78 (0.56–5.62) | 0.33 |
| 13 to 14.9 | 90.0 | 21 | 1.40 (0.51–3.82) | 0.52 | 0.79 (0.19–3.22) | 0.74 |
| 15 to 17.9 | 98.0 | 29 | 0.71 (0.26–1.97) | 0.51 | 0.40 (0.11–1.47) | 0.17 |
| WC/HT (cm/m) | ||||||
| 5 to 6.9 | 57.63 | 9 | 0.73 (0.14–3.78) | 0.71 | 0.80 (0.14–4.74) | 0.81 |
| 7 to 8.9 | 56.77 | 14 | 0.86 (0.25–2.92) | 0.81 | 1.45 (0.30–7.00) | 0.64 |
| 9 to 10.9 | 60.52 | 18 | 1.32 (0.47–3.73) | 0.60 | 1.25 (0.35–4.49) | 0.73 |
| 11 to 12.9 | 60.56 | 19 | 1.03 (0.38–2.74) | 0.96 | 1.28 (0.39–4.24) | 0.68 |
| 13 to 14.9 | 55.93 | 21 | 1.40 (0.51–3.82) | 0.52 | 0.92 (0.25–3.31) | 0.89 |
| 15 to 17.9 | 57.54 | 25 | 0.65 (0.21–1.98) | 0.45 | 0.35 (0.09–1.45) | 0.15 |
| WC/HT2 (cm/m2) | ||||||
| 5 to 6.9 | 49.43 | 9 | # | # | ||
| 7 to 8.9 | 43.83 | 14 | 0.86 (0.25–2.92) | 0.81 | 1.35 (0.31–5.94) | 0.69 |
| 9 to 10.9 | 42.44 | 18 | 0.98 (0.33–2.92) | 0.98 | 0.81 (0.22–2.98) | 0.75 |
| 11 to 12.9 | 39.15 | 19 | 1.03 (0.38–2.74) | 0.96 | 1.28 (0.39–4.24) | 0.68 |
| 13 to 14.9 | 35.84 | 21 | 1.40 (0.51–3.82) | 0.52 | 1.02 (0.31–3.35) | 0.98 |
| 15 to 17.9 | 33.87 | 25 | 0.45 (0.13–1.58) | 0.21 | 0.26 (0.07–1.07) | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jáuregui-Ulloa, E.; Gaytán-González, A.; Elizalde-Villarreal, M.; González-Navarro, E.; Ocampo-Chavarría, A.; López-Taylor, J. Waist Circumference Is Not Associated with Impaired Fasting Blood Glucose in a Sample of Mexican Children and Teenagers: Results from a State Screening Program. Children 2021, 8, 172. https://doi.org/10.3390/children8030172
Jáuregui-Ulloa E, Gaytán-González A, Elizalde-Villarreal M, González-Navarro E, Ocampo-Chavarría A, López-Taylor J. Waist Circumference Is Not Associated with Impaired Fasting Blood Glucose in a Sample of Mexican Children and Teenagers: Results from a State Screening Program. Children. 2021; 8(3):172. https://doi.org/10.3390/children8030172
Chicago/Turabian StyleJáuregui-Ulloa, Edtna, Alejandro Gaytán-González, Mayra Elizalde-Villarreal, Esmeralda González-Navarro, Alberto Ocampo-Chavarría, and Juan López-Taylor. 2021. "Waist Circumference Is Not Associated with Impaired Fasting Blood Glucose in a Sample of Mexican Children and Teenagers: Results from a State Screening Program" Children 8, no. 3: 172. https://doi.org/10.3390/children8030172