Ultra-Processed Food, Reward System and Childhood Obesity
Abstract
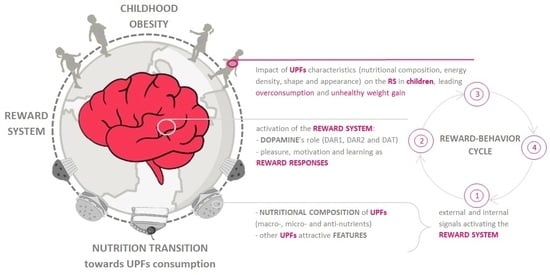
:1. Introduction
2. Methods
3. Childhood Obesity
4. Feeding Regulation and Brain Reward System
4.1. Feeding Regulation
4.2. Brain Reward System
5. Ultra Processed Food in Childhood Obesity
6. Ultra-Processed Foods and Reward System in Children
6.1. Nutritional Factors Characterizing UPFs with a Potential Impact on the Reward System and Predisposing toward Overconsumption
6.2. Effect of UPFs on the Reward System, Promoting Excessive Energy Consumption
6.3. “Addictive-like Behaviour” and Ultra-Processed Foods: The Debate in the Literature on Whether or Not It Is Possible to Talk about “Addiction to Ultra-Processed Foods”
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 December 2022).
- Sahoo, K.; Sahoo, B.; Choudhury, A.; Sofi, N.; Kumar, R.; Bhadoria, A. Childhood Obesity: Causes and Consequences. J. Fam. Med. Prim. Care 2015, 4, 187. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-Processed Foods: What They Are and How to Identify Them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Huffman, S.L.; Piwoz, E.G.; Vosti, S.A.; Dewey, K.G. Babies, Soft Drinks and Snacks: A Concern in Low- and Middle-Income Countries? Matern. Child. Nutr. 2014, 10, 562–574. [Google Scholar] [CrossRef] [Green Version]
- Rousham, E.K.; Goudet, S.; Markey, O.; Griffiths, P.; Boxer, B.; Carroll, C.; Petherick, E.S.; Pradeilles, R. Unhealthy Food and Beverage Consumption in Children and Risk of Overweight and Obesity: A Systematic Review and Meta-Analysis. Adv. Nutr. 2022, 13, 1669–1696. [Google Scholar] [CrossRef] [PubMed]
- Pries, A.M.; Filteau, S.; Ferguson, E.L. Snack Food and Beverage Consumption and Young Child Nutrition in Low- and Middle-Income Countries: A Systematic Review. Matern. Child. Nutr. 2019, 15 (Suppl. 4), e12729. [Google Scholar] [CrossRef] [Green Version]
- GBD 2017 Diet Collaborators Health Effects of Dietary Risks in 195 Countries, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [CrossRef] [Green Version]
- Costa, C.S.; Rauber, F.; Leffa, P.S.; Sangalli, C.N.; Campagnolo, P.D.B.; Vitolo, M.R. Ultra-Processed Food Consumption and Its Effects on Anthropometric and Glucose Profile: A Longitudinal Study during Childhood. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 177–184. [Google Scholar] [CrossRef]
- Blundell, J.; de Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; van der Knaap, H.; et al. Appetite Control: Methodological Aspects of the Evaluation of Foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef] [Green Version]
- Amin, T.; Mercer, J.G. Hunger and Satiety Mechanisms and Their Potential Exploitation in the Regulation of Food Intake. Curr. Obes. Rep. 2016, 5, 106–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weltens, N.; Zhao, D.; Van Oudenhove, L. Where Is the Comfort in Comfort Foods? Mechanisms Linking Fat Signaling, Reward, and Emotion. Neurogastroenterol. Motil. 2014, 26, 303–315. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M.L. Pleasure Systems in the Brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, I.; Berridge, K.C. “Liking” and “Wanting” in Eating and Food Reward: Brain Mechanisms and Clinical Implications. Physiol. Behav. 2020, 227, 113152. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews-Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Bradwisch, S.A.; Smith, E.M.; Mooney, C.; Scaccia, D. Obesity in Children and Adolescents: An Overview. Nursing 2020, 50, 60–66. [Google Scholar] [CrossRef]
- Taghizadeh, S.; Farhangi, M.A. The Effectiveness of Pediatric Obesity Prevention Policies: A Comprehensive Systematic Review and Dose–Response Meta-Analysis of Controlled Clinical Trials. J. Transl. Med. 2020, 18, 480. [Google Scholar] [CrossRef] [PubMed]
- Kansra, A.R.; Lakkunarajah, S.; Jay, M.S. Childhood and Adolescent Obesity: A Review. Front. Pediatr. 2021, 8, 581461. [Google Scholar] [CrossRef] [PubMed]
- Epicentro-ISS Istituto Superiore di Sanità Sistema Di Sorveglianza Okkio Alla SALUTE 2019. Available online: https://www.epicentro.iss.it/okkioallasalute/indagine-2019-dati (accessed on 2 January 2023).
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128·9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [CrossRef] [Green Version]
- Geserick, M.; Vogel, M.; Gausche, R.; Lipek, T.; Spielau, U.; Keller, E.; Pfäffle, R.; Kiess, W.; Körner, A. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N. Engl. J. Med. 2018, 379, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Rokholm, B.; Baker, J.L.; Sørensen, T.I.A. The Levelling off of the Obesity Epidemic since the Year 1999--a Review of Evidence and Perspectives. Obes. Rev. 2010, 11, 835–846. [Google Scholar] [CrossRef]
- Qasim, A.; Turcotte, M.; de Souza, R.J.; Samaan, M.C.; Champredon, D.; Dushoff, J.; Speakman, J.R.; Meyre, D. On the Origin of Obesity: Identifying the Biological, Environmental and Cultural Drivers of Genetic Risk among Human Populations: Evolution and Genetic Risk of Obesity. Obes. Rev. 2018, 19, 121–149. [Google Scholar] [CrossRef] [PubMed]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in Children and Adolescents: Epidemiology, Causes, Assessment, and Management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Roberto, C.A.; Swinburn, B.; Hawkes, C.; Huang, T.T.-K.; Costa, S.A.; Ashe, M.; Zwicker, L.; Cawley, J.H.; Brownell, K.D. Patchy Progress on Obesity Prevention: Emerging Examples, Entrenched Barriers, and New Thinking. Lancet 2015, 385, 2400–2409. [Google Scholar] [CrossRef]
- Brown, C.L.; Halvorson, E.E.; Cohen, G.M.; Lazorick, S.; Skelton, J.A. Addressing Childhood Obesity: Opportunities for Prevention. Pediatr. Clin. N. Am. 2015, 62, 1241–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahumud, R.A.; Sahle, B.W.; Owusu-Addo, E.; Chen, W.; Morton, R.L.; Renzaho, A.M.N. Association of Dietary Intake, Physical Activity, and Sedentary Behaviours with Overweight and Obesity among 282,213 Adolescents in 89 Low and Middle Income to High-Income Countries. Int. J. Obes. 2021, 45, 2404–2418. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Mu, M.; Liu, K.; He, Y. Screen Time and Childhood Overweight/Obesity: A Systematic Review and Meta-Analysis. Child. Care Health Dev. 2019, 45, 744–753. [Google Scholar] [CrossRef]
- Sadeghirad, B.; Duhaney, T.; Motaghipisheh, S.; Campbell, N.R.C.; Johnston, B.C. Influence of Unhealthy Food and Beverage Marketing on Children’s Dietary Intake and Preference: A Systematic Review and Meta-Analysis of Randomized Trials: Meta-Analysis of Unhealthy Food and Beverage Marketing. Obes. Rev. 2016, 17, 945–959. [Google Scholar] [CrossRef]
- Poorolajal, J.; Sahraei, F.; Mohamdadi, Y.; Doosti-Irani, A.; Moradi, L. Behavioral Factors Influencing Childhood Obesity: A Systematic Review and Meta-Analysis. Obes. Res. Clin. Pract. 2020, 14, 109–118. [Google Scholar] [CrossRef]
- Kavey, R.-E.W. How Sweet It Is: Sugar-Sweetened Beverage Consumption, Obesity, and Cardiovascular Risk in Childhood. J. Am. Diet. Assoc. 2010, 110, 1456–1460. [Google Scholar] [CrossRef]
- Dereń, K.; Weghuber, D.; Caroli, M.; Koletzko, B.; Thivel, D.; Frelut, M.-L.; Socha, P.; Grossman, Z.; Hadjipanayis, A.; Wyszyńska, J.; et al. Consumption of Sugar-Sweetened Beverages in Paediatric Age: A Position Paper of the European Academy of Paediatrics and the European Childhood Obesity Group. Ann. Nutr. Metab. 2019, 74, 296–302. [Google Scholar] [CrossRef]
- Abbasalizad Farhangi, M.; Mohammadi Tofigh, A.; Jahangiri, L.; Nikniaz, Z.; Nikniaz, L. Sugar-Sweetened Beverages Intake and the Risk of Obesity in Children: An Updated Systematic Review and Dose-Response Meta-Analysis. Pediatr. Obes. 2022, 17, e12914. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Cena, H.; Magenes, V.C.; Vincenti, A.; Comola, G.; Beretta, A.; Di Napoli, I.; Zuccotti, G. Sugar-Sweetened Beverages and Metabolic Risk in Children and Adolescents with Obesity: A Narrative Review. Nutrients 2023, 15, 702. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Fakhouri, T.H.; Hales, C.M.; Fryar, C.D.; Li, X.; Freedman, D.S. Prevalence of Obesity Among Youths by Household Income and Education Level of Head of Household—United States 2011–2014. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.S.; Barlow, S.E.; Rao, G.; Inge, T.H.; Hayman, L.L.; Steinberger, J.; Urbina, E.M.; Ewing, L.J.; Daniels, S.R. Severe Obesity in Children and Adolescents: Identification, Associated Health Risks, and Treatment Approaches: A Scientific Statement From the American Heart Association. Circulation 2013, 128, 1689–1712. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M.; Butcher, K.F. (Kristin F. Childhood Obesity: Trends and Potential Causes. Future Child. 2006, 16, 19–45. [Google Scholar] [CrossRef]
- Cena, H.; Fiechtner, L.; Vincenti, A.; Magenes, V.C.; De Giuseppe, R.; Manuelli, M.; Zuccotti, G.V.; Calcaterra, V. COVID-19 Pandemic as Risk Factors for Excessive Weight Gain in Pediatrics: The Role of Changes in Nutrition Behavior. A Narrative Review. Nutrients 2021, 13, 4255. [Google Scholar] [CrossRef]
- Galler, A.; Röbl, M.; Prinz, N.; Dannemann, A.; Gellhaus, I.; Kapellen, T.; Linke, S.; Schauerte, G.; Stein, R.; Weghuber, D.; et al. Weight Development in Children and Adolescents with Obesity during the COVID-19 Pandemic. Dtsch. Ärzteblatt Int. 2022, 119, 302–303. [Google Scholar] [CrossRef]
- Marcovecchio, M.L.; Gorman, S.; Watson, L.P.E.; Dunger, D.B.; Beardsall, K. Catch-Up Growth in Children Born Small for Gestational Age Related to Body Composition and Metabolic Risk at Six Years of Age in the UK. Horm. Res. Paediatr. 2020, 93, 119–127. [Google Scholar] [CrossRef]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, Treatment and Prevention of Pediatric Obesity: Consensus Position Statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Indrio, F.; Martini, S.; Francavilla, R.; Corvaglia, L.; Cristofori, F.; Mastrolia, S.A.; Neu, J.; Rautava, S.; Russo Spena, G.; Raimondi, F.; et al. Epigenetic Matters: The Link between Early Nutrition, Microbiome, and Long-Term Health Development. Front. Pediatr. 2017, 5, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Simple Tests for the Diagnosis of Childhood Obesity: A Systematic Review and Meta-Analysis. Obes. Rev. 2016, 17, 1301–1315. [Google Scholar] [CrossRef] [Green Version]
- Calcaterra, V.; Rossi, V.; Mari, A.; Casini, F.; Bergamaschi, F.; Zuccotti, G.V.; Fabiano, V. Medical Treatment of Weight Loss in Children and Adolescents with Obesity. Pharmacol. Res. 2022, 185, 106471. [Google Scholar] [CrossRef] [PubMed]
- Jebeile, H.; Cardel, M.I.; Kyle, T.K.; Jastreboff, A.M. Addressing Psychosocial Health in the Treatment and Care of Adolescents with Obesity. Obesity 2021, 29, 1413–1422. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended International (IOTF) Body Mass Index Cut-Offs for Thinness, Overweight and Obesity: Extended International BMI Cut-Offs. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- WHO Child Growth Standards. Available online: https://www.who.int/tools/child-growth-standards (accessed on 2 January 2023).
- Horesh, A.; Tsur, A.M.; Bardugo, A.; Twig, G. Adolescent and Childhood Obesity and Excess Morbidity and Mortality in Young Adulthood-a Systematic Review. Curr. Obes. Rep. 2021, 10, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Pulgarón, E.R. Childhood Obesity: A Review of Increased Risk for Physical and Psychological Comorbidities. Clin. Ther. 2013, 35, A18–A32. [Google Scholar] [CrossRef] [Green Version]
- Ferrante, A.W. Obesity-Induced Inflammation: A Metabolic Dialogue in the Language of Inflammation. J. Intern. Med. 2007, 262, 408–414. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O.; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals From the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Calcaterra, V.; Klersy, C.; Muratori, T.; Telli, S.; Caramagna, C.; Scaglia, F.; Cisternino, M.; Larizza, D. Prevalence of Metabolic Syndrome (MS) in Children and Adolescents with Varying Degrees of Obesity. Clin. Endocrinol. 2008, 68, 868–872. [Google Scholar] [CrossRef] [PubMed]
- De Leonibus, C.; Marcovecchio, M.L.; Chiarelli, F. Update on Statural Growth and Pubertal Development in Obese Children. Pediatr. Rep. 2012, 4, e35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witchel, S.F.; Burghard, A.C.; Tao, R.H.; Oberfield, S.E. The Diagnosis and Treatment of PCOS in Adolescents: An Update. Curr. Opin. Pediatr. 2019, 31, 562–569. [Google Scholar] [CrossRef]
- Marcus, C.L.; Brooks, L.J.; Ward, S.D.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Lehmann, C.; Schechter, M.S.; Sheldon, S.; et al. Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome. Pediatrics 2012, 130, e714–e755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rankin, J.; Matthews, L.; Cobley, S.; Han, A.; Sanders, R.; Wiltshire, H.D.; Baker, J.S. Psychological Consequences of Childhood Obesity: Psychiatric Comorbidity and Prevention. Adolesc. Health Med. Ther. 2016, 7, 125–146. [Google Scholar] [CrossRef] [Green Version]
- Topçu, S.; Orhon, F.Ş.; Tayfun, M.; Uçaktürk, S.A.; Demirel, F. Anxiety, Depression and Self-Esteem Levels in Obese Children: A Case-Control Study. J. Pediatr. Endocrinol. Metab. 2016, 29, 254. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Barlow, S.; Bouchard, C.; Catalano, P.M.; Hsia, D.S.; Inge, T.H.; Lovelady, C.; Raynor, H.; Redman, L.M.; Staiano, A.E.; et al. An Evolving Scientific Basis for the Prevention and Treatment of Pediatric Obesity. Int. J. Obes. 2014, 38, 887–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, T.; Moore, T.H.; Hooper, L.; Gao, Y.; Zayegh, A.; Ijaz, S.; Elwenspoek, M.; Foxen, S.C.; Magee, L.; O’Malley, C.; et al. Interventions for Preventing Obesity in Children. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.; Scarborough, P.; Rayner, M.; Briggs, A.D.M. Should We Tax Unhealthy Food and Drink? Proc. Nutr. Soc. 2018, 77, 314–320. [Google Scholar] [CrossRef]
- Figlewicz, D.P.; Sipols, A.J. Energy Regulatory Signals and Food Reward. Pharmacol. Biochem. Behav. 2010, 97, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Lutter, M.; Nestler, E.J. Homeostatic and Hedonic Signals Interact in the Regulation of Food Intake. J. Nutr. 2009, 139, 629–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, M.A.; Stuber, G.D. Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell. Metabolism 2018, 27, 42–56. [Google Scholar] [CrossRef]
- Kenny, P.J. Common Cellular and Molecular Mechanisms in Obesity and Drug Addiction. Nat. Rev. Neurosci. 2011, 12, 638–651. [Google Scholar] [CrossRef]
- Berridge, K.C. Food Reward: Brain Substrates of Wanting and Liking. Neurosci. Biobehav. Rev. 1996, 20, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hoebel, B.G.; Teitelbaum, P. Hypothalamic Control of Feeding and Self-Stimulation. Science 1962, 135, 375–377. [Google Scholar] [CrossRef]
- Margules, D.L.; Olds, J. Identical “Feeding” and “Rewarding” Systems in the Lateral Hypothalamus of Rats. Science 1962, 135, 374–375. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A. Dopamine, Learning and Motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef]
- Corbett, D.; Wise, R.A. Intracranial Self-Stimulation in Relation to the Ascending Dopaminergic Systems of the Midbrain: A Moveable Electrode Mapping Study. Brain Res. 1980, 185, 1–15. [Google Scholar] [CrossRef]
- Schultz, W. Getting Formal with Dopamine and Reward. Neuron 2002, 36, 241–263. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.; Nevet, A.; Arkadir, D.; Vaadia, E.; Bergman, H. Midbrain Dopamine Neurons Encode Decisions for Future Action. Nat. Neurosci. 2006, 9, 1057–1063. [Google Scholar] [CrossRef]
- Castro, D.C.; Cole, S.L.; Berridge, K.C. Lateral Hypothalamus, Nucleus Accumbens, and Ventral Pallidum Roles in Eating and Hunger: Interactions between Homeostatic and Reward Circuitry. Front. Syst. Neurosci. 2015, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saper, C.B.; Chou, T.C.; Elmquist, J.K. The Need to Feed. Neuron 2002, 36, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Blundell, J.E.; Lawton, C.L.; Hill, A.J. Mechanisms of Appetite Control and Their Abnormalities in Obese Patients. Horm. Res. 1993, 39, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Halford, J.C.G.; Harrold, J.A. Satiety-Enhancing Products for Appetite Control: Science and Regulation of Functional Foods for Weight Management. Proc. Nutr. Soc. 2012, 71, 350–362. [Google Scholar] [CrossRef]
- Davis, J. Hunger, Ghrelin and the Gut. Brain Res. 2018, 1693, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Van Kleef, E.; Van Trijp, J.C.M.; Van Den Borne, J.J.G.C.; Zondervan, C. Successful Development of Satiety Enhancing Food Products: Towards a Multidisciplinary Agenda of Research Challenges. Crit. Rev. Food Sci. Nutr. 2012, 52, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Demori, I.; Grasselli, E. Stress-Related Weight Gain: Mechanisms Involving Feeding Behavior, Metabolism, Gut Microbiota and Inflammation. J. Nutr. Food Sci. 2016, 6, 457. [Google Scholar] [CrossRef] [Green Version]
- Mietus-Snyder, M.L.; Lustig, R.H. Childhood Obesity: Adrift in the “Limbic Triangle”. Annu. Rev. Med. 2008, 59, 147–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, G.; Wardle, J. Perceived Effects of Stress on Food Choice. Physiol. Behav. 1999, 66, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.G.; Cohen, P.; Kasen, S.; Brook, J.S. Childhood Adversities Associated with Risk for Eating Disorders or Weight Problems during Adolescence or Early Adulthood. Am. J. Psychiatry 2002, 159, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Roemmich, J.N.; Wright, S.M.; Epstein, L.H. Dietary Restraint and Stress-Induced Snacking in Youth. Obes. Res. 2002, 10, 1120–1126. [Google Scholar] [CrossRef]
- Espel-Huynh, H.M.; Muratore, A.F.; Lowe, M.R. A Narrative Review of the Construct of Hedonic Hunger and Its Measurement by the Power of Food Scale: Hedonic Hunger Review. Obes. Sci. Pract. 2018, 4, 238–249. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Alonso, M.; Woods, S.C.; Pelchat, M.; Grigson, P.S.; Stice, E.; Farooqi, S.; Khoo, C.S.; Mattes, R.D.; Beauchamp, G.K. Food Reward System: Current Perspectives and Future Research Needs. Nutr. Rev. 2015, 73, 296–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, G.; Adan, R.A.H.; Belot, M.; Brunstrom, J.M.; de Graaf, K.; Dickson, S.L.; Hare, T.; Maier, S.; Menzies, J.; Preissl, H.; et al. The Determinants of Food Choice. Proc. Nutr. Soc. 2017, 76, 316–327. [Google Scholar] [CrossRef] [Green Version]
- Palmiter, R.D. Is Dopamine a Physiologically Relevant Mediator of Feeding Behavior? Trends Neurosci. 2007, 30, 375–381. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Gainetdinov, R.R. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tritsch, N.X.; Sabatini, B.L. Dopaminergic Modulation of Synaptic Transmission in Cortex and Striatum. Neuron 2012, 76, 33–50. [Google Scholar] [CrossRef] [Green Version]
- Pothos, E.N.; Davila, V.; Sulzer, D. Presynaptic Recording of Quanta from Midbrain Dopamine Neurons and Modulation of the Quantal Size. J. Neurosci. 1998, 18, 4106–4118. [Google Scholar] [CrossRef] [Green Version]
- Cass, W.A.; Zahniser, N.R. Potassium Channel Blockers Inhibit D2 Dopamine, but Not A1 Adenosine, Receptor-Mediated Inhibition of Striatal Dopamine Release. J. Neurochem. 1991, 57, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Congar, P.; Bergevin, A.; Trudeau, L.-E. D2 Receptors Inhibit the Secretory Process Downstream From Calcium Influx in Dopaminergic Neurons: Implication of K + Channels. J. Neurophysiol. 2002, 87, 1046–1056. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.M.; Kenny, P.J. Dopamine D2 Receptors in Addiction-like Reward Dysfunction and Compulsive Eating in Obese Rats. Nat. Neurosci. 2010, 13, 635–641. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Buck, D.C.; Yang, R.; Macey, T.A.; Neve, K.A. Dopamine D2 Receptor Stimulation of Mitogen-Activated Protein Kinases Mediated by Cell Type-Dependent Transactivation of Receptor Tyrosine Kinases. J. Neurochem. 2005, 93, 899–909. [Google Scholar] [CrossRef]
- Stice, E.; Yokum, S.; Zald, D.; Dagher, A. Dopamine-Based Reward Circuitry Responsivity, Genetics, and Overeating. In Behavioral Neurobiology of Eating Disorders; Adan, R.A.H., Kaye, W.H., Eds.; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6, pp. 81–93. ISBN 978-3-642-15130-9. [Google Scholar]
- Baik, J.-H. Dopamine Signaling in Reward-Related Behaviors. Front. Neural Circuits 2013, 7, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozuka, C.; Kaname, T.; Shimizu-Okabe, C.; Takayama, C.; Tsutsui, M.; Matsushita, M.; Abe, K.; Masuzaki, H. Impact of Brown Rice-Specific γ-Oryzanol on Epigenetic Modulation of Dopamine D2 Receptors in Brain Striatum in High-Fat-Diet-Induced Obesity in Mice. Diabetologia 2017, 60, 1502–1511. [Google Scholar] [CrossRef] [Green Version]
- Hommel, J.D.; Trinko, R.; Sears, R.M.; Georgescu, D.; Liu, Z.-W.; Gao, X.-B.; Thurmon, J.J.; Marinelli, M.; DiLeone, R.J. Leptin Receptor Signaling in Midbrain Dopamine Neurons Regulates Feeding. Neuron 2006, 51, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooqi, I.S.; Bullmore, E.; Keogh, J.; Gillard, J.; O’Rahilly, S.; Fletcher, P.C. Leptin Regulates Striatal Regions and Human Eating Behavior. Science 2007, 317, 1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Wang, G.-J.; Baler, R.D. Reward, Dopamine and the Control of Food Intake: Implications for Obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abizaid, A.; Liu, Z.-W.; Andrews, Z.B.; Shanabrough, M.; Borok, E.; Elsworth, J.D.; Roth, R.H.; Sleeman, M.W.; Picciotto, M.R.; Tschöp, M.H.; et al. Ghrelin Modulates the Activity and Synaptic Input Organization of Midbrain Dopamine Neurons While Promoting Appetite. J. Clin. Investig. 2006, 116, 3229–3239. [Google Scholar] [CrossRef]
- Khanh, D.V.; Choi, Y.-H.; Moh, S.H.; Kinyua, A.W.; Kim, K.W. Leptin and Insulin Signaling in Dopaminergic Neurons: Relationship between Energy Balance and Reward System. Front. Psychol. 2014, 5, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruijnzeel, A.W.; Corrie, L.W.; Rogers, J.A.; Yamada, H. Effects of Insulin and Leptin in the Ventral Tegmental Area and Arcuate Hypothalamic Nucleus on Food Intake and Brain Reward Function in Female Rats. Behav. Brain Res. 2011, 219, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Mebel, D.M.; Wong, J.C.; Dong, Y.J.; Borgland, S.L. Insulin in the Ventral Tegmental Area Reduces Hedonic Feeding and Suppresses Dopamine Concentration via Increased Reuptake: Insulin Attenuates Somatodendritic Dopamine. Eur. J. Neurosci. 2012, 36, 2336–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Könner, A.C.; Hess, S.; Tovar, S.; Mesaros, A.; Sánchez-Lasheras, C.; Evers, N.; Verhagen, L.A.W.; Brönneke, H.S.; Kleinridders, A.; Hampel, B.; et al. Role for Insulin Signaling in Catecholaminergic Neurons in Control of Energy Homeostasis. Cell. Metab. 2011, 13, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.R.; Haas, E.; Barton, M. Gender Differences of Cardiovascular Disease: New Perspectives for Estrogen Receptor Signaling. Hypertension 2006, 47, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Sienkiewicz-Jarosz, H.; Scinska, A.; Swiecicki, L.; Lipczynska-Lojkowska, W.; Kuran, W.; Ryglewicz, D.; Kolaczkowski, M.; Samochowiec, J.; Bienkowski, P. Sweet Liking in Patients with Parkinson’s Disease. J. Neurol. Sci. 2013, 329, 17–22. [Google Scholar] [CrossRef]
- Ireland, J.D.; Møller, A. LanguaL Food Description: A Learning Process. Eur. J. Clin. Nutr. 2010, 64 (Suppl. 3), S44–S48. [Google Scholar] [CrossRef] [Green Version]
- Becker, W.; Møller, A.; Ireland, J.; Roe, M.; Unwin, I.; Pakkala, H. Proposal for Structure and Detail of a EuroFIR Standard on Food Composition Data. II: Technical Annex. In Danish Food Information; European Food Information Resource Network: Brussels, Belgium, 2008. [Google Scholar]
- Lauria, F.; Dello Russo, M.; Formisano, A.; De Henauw, S.; Hebestreit, A.; Hunsberger, M.; Krogh, V.; Intemann, T.; Lissner, L.; Molnar, D.; et al. Ultra-Processed Foods Consumption and Diet Quality of European Children, Adolescents and Adults: Results from the I.Family Study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3031–3043. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; de Castro, I.R.R.; Cannon, G. Increasing Consumption of Ultra-Processed Foods and Likely Impact on Human Health: Evidence from Brazil. Public Health Nutr. 2011, 14, 5–13. [Google Scholar] [CrossRef]
- Parra, D.C.; da Costa-Louzada, M.L.; Moubarac, J.-C.; Bertazzi-Levy, R.; Khandpur, N.; Cediel, G.; Monteiro, C.A. Association between Ultra-Processed Food Consumption and the Nutrient Profile of the Colombian Diet in 2005. Salud Publica Mex. 2019, 61, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Petrus, R.R.; do Amaral Sobral, P.J.; Tadini, C.C.; Gonçalves, C.B. The NOVA Classification System: A Critical Perspective in Food Science. Trends Food Sci. Technol. 2021, 116, 603–608. [Google Scholar] [CrossRef]
- Marino, M.; Puppo, F.; Del Bo’, C.; Vinelli, V.; Riso, P.; Porrini, M.; Martini, D. A Systematic Review of Worldwide Consumption of Ultra-Processed Foods: Findings and Criticisms. Nutrients 2021, 13, 2778. [Google Scholar] [CrossRef] [PubMed]
- Slimani, N.; Deharveng, G.; Southgate, D.A.T.; Biessy, C.; Chajès, V.; van Bakel, M.M.E.; Boutron-Ruault, M.C.; McTaggart, A.; Grioni, S.; Verkaik-Kloosterman, J.; et al. Contribution of Highly Industrially Processed Foods to the Nutrient Intakes and Patterns of Middle-Aged Populations in the European Prospective Investigation into Cancer and Nutrition Study. Eur. J. Clin. Nutr. 2009, 63 (Suppl. 4), S206–S225. [Google Scholar] [CrossRef] [PubMed]
- Davidou, S.; Christodoulou, A.; Fardet, A.; Frank, K. The Holistico-Reductionist Siga Classification According to the Degree of Food Processing: An Evaluation of Ultra-Processed Foods in French Supermarkets. Food Funct. 2020, 11, 2026–2039. [Google Scholar] [CrossRef]
- Moodie, R.; Stuckler, D.; Monteiro, C.; Sheron, N.; Neal, B.; Thamarangsi, T.; Lincoln, P.; Casswell, S. Lancet NCD Action Group Profits and Pandemics: Prevention of Harmful Effects of Tobacco, Alcohol, and Ultra-Processed Food and Drink Industries. Lancet 2013, 381, 670–679. [Google Scholar] [CrossRef]
- Ronto, R.; Wu, J.H.; Singh, G.M. The Global Nutrition Transition: Trends, Disease Burdens and Policy Interventions. Public Health Nutr. 2018, 21, 2267–2270. [Google Scholar] [CrossRef] [Green Version]
- Popkin, B.M. Relationship between Shifts in Food System Dynamics and Acceleration of the Global Nutrition Transition. Nutr. Rev. 2017, 75, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Kelly, A.L.; Baugh, M.E.; Oster, M.E.; DiFeliceantonio, A.G. The Impact of Caloric Availability on Eating Behavior and Ultra-Processed Food Reward. Appetite 2022, 178, 106274. [Google Scholar] [CrossRef]
- Baraldi, L.G.; Martinez Steele, E.; Canella, D.S.; Monteiro, C.A. Consumption of Ultra-Processed Foods and Associated Sociodemographic Factors in the USA between 2007 and 2012: Evidence from a Nationally Representative Cross-Sectional Study. BMJ Open. 2018, 8, e020574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moubarac, J.-C.; Batal, M.; Martins, A.P.B.; Claro, R.; Levy, R.B.; Cannon, G.; Monteiro, C. Processed and Ultra-Processed Food Products: Consumption Trends in Canada from 1938 to 2011. Can. J. Diet. Pract. Res. 2014, 75, 15–21. [Google Scholar] [CrossRef]
- Neri, D.; Steele, E.M.; Khandpur, N.; Cediel, G.; Zapata, M.E.; Rauber, F.; Marrón-Ponce, J.A.; Machado, P.; Costa Louzada, M.L.; Andrade, G.C.; et al. Ultraprocessed Food Consumption and Dietary Nutrient Profiles Associated with Obesity: A Multicountry Study of Children and Adolescents. Obes. Rev. 2022, 23, 3387. [Google Scholar] [CrossRef] [PubMed]
- Martines, R.M.; Machado, P.P.; Neri, D.A.; Levy, R.B.; Rauber, F. Association between Watching TV Whilst Eating and Children’s Consumption of Ultraprocessed Foods in United Kingdom. Matern. Child. Nutr. 2019, 15, e12819. [Google Scholar] [CrossRef] [PubMed]
- Marrón-Ponce, J.A.; Sánchez-Pimienta, T.G.; da Costa Louzada, M.L.; Batis, C. Energy Contribution of NOVA Food Groups and Sociodemographic Determinants of Ultra-Processed Food Consumption in the Mexican Population. Public Health Nutr. 2018, 21, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cediel, G.; Reyes, M.; da Costa Louzada, M.L.; Martinez Steele, E.; Monteiro, C.A.; Corvalán, C.; Uauy, R. Ultra-Processed Foods and Added Sugars in the Chilean Diet (2010). Public Health Nutr. 2018, 21, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, E.; Esposito, S.; Costanzo, S.; Di Castelnuovo, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Bonaccio, M. Ultra-Processed Food Consumption and Its Correlates among Italian Children, Adolescents and Adults from the Italian Nutrition & Health Survey (INHES) Cohort Study. Public Health Nutr. 2021, 24, 6258–6271. [Google Scholar] [CrossRef] [PubMed]
- Camara, S.; de Lauzon-Guillain, B.; Heude, B.; Charles, M.-A.; Botton, J.; Plancoulaine, S.; Forhan, A.; Saurel-Cubizolles, M.-J.; Dargent-Molina, P.; Lioret, S.; et al. Multidimensionality of the Relationship between Social Status and Dietary Patterns in Early Childhood: Longitudinal Results from the French EDEN Mother-Child Cohort. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 122. [Google Scholar] [CrossRef] [Green Version]
- Khandpur, N.; Neri, D.A.; Monteiro, C.; Mazur, A.; Frelut, M.-L.; Boyland, E.; Weghuber, D.; Thivel, D. Ultra-Processed Food Consumption among the Paediatric Population: An Overview and Call to Action from the European Childhood Obesity Group. Ann. Nutr. Metab. 2020, 76, 109–113. [Google Scholar] [CrossRef]
- Martini, D.; Godos, J.; Bonaccio, M.; Vitaglione, P.; Grosso, G. Ultra-Processed Foods and Nutritional Dietary Profile: A Meta-Analysis of Nationally Representative Samples. Nutrients 2021, 13, 3390. [Google Scholar] [CrossRef]
- Schiestl, E.T.; Rios, J.M.; Parnarouskis, L.; Cummings, J.R.; Gearhardt, A.N. A Narrative Review of Highly Processed Food Addiction across the Lifespan. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110152. [Google Scholar] [CrossRef]
- De Amicis, R.; Mambrini, S.P.; Pellizzari, M.; Foppiani, A.; Bertoli, S.; Battezzati, A.; Leone, A. Ultra-Processed Foods and Obesity and Adiposity Parameters among Children and Adolescents: A Systematic Review. Eur. J. Nutr. 2022, 61, 2297–2311. [Google Scholar] [CrossRef]
- Birch, L.L.; Doub, A.E. Learning to Eat: Birth to Age 2 y. Am. J. Clin. Nutr. 2014, 99, 723S–728S. [Google Scholar] [CrossRef] [Green Version]
- Luque, V.; Escribano, J.; Closa-Monasterolo, R.; Zaragoza-Jordana, M.; Ferré, N.; Grote, V.; Koletzko, B.; Totzauer, M.; Verduci, E.; ReDionigi, A.; et al. Unhealthy Dietary Patterns Established in Infancy Track to Mid-Childhood: The EU Childhood Obesity Project. J. Nutr. 2018, 148, 752–759. [Google Scholar] [CrossRef] [Green Version]
- Gordon, E.L.; Lent, M.R.; Merlo, L.J. The Effect of Food Composition and Behavior on Neurobiological Response to Food: A Review of Recent Research. Curr. Nutr. Rep. 2020, 9, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; da Costa Louzada, M.L.; Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System 2019. Available online: https://www.fao.org/3/ca5644en/ca5644en.pdf (accessed on 18 December 2022).
- Rauber, F.; Campagnolo, P.D.B.; Hoffman, D.J.; Vitolo, M.R. Consumption of Ultra-Processed Food Products and Its Effects on Children’s Lipid Profiles: A Longitudinal Study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 116–122. [Google Scholar] [CrossRef] [PubMed]
- da Costa Louzada, M.L.; Baraldi, L.G.; Steele, E.M.; Martins, A.P.B.; Canella, D.S.; Moubarac, J.-C.; Levy, R.B.; Cannon, G.; Afshin, A.; Imamura, F.; et al. Consumption of Ultra-Processed Foods and Obesity in Brazilian Adolescents and Adults. Prev. Med. 2015, 81, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Mendonça, R.d.D.; Pimenta, A.M.; Gea, A.; de la Fuente-Arrillaga, C.; Martinez-Gonzalez, M.A.; Lopes, A.C.S.; Bes-Rastrollo, M. Ultraprocessed Food Consumption and Risk of Overweight and Obesity: The University of Navarra Follow-Up (SUN) Cohort Study. Am. J. Clin. Nutr. 2016, 104, 1433–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Escamilla, R.; Obbagy, J.E.; Altman, J.M.; Essery, E.V.; McGrane, M.M.; Wong, Y.P.; Spahn, J.M.; Williams, C.L. Dietary Energy Density and Body Weight in Adults and Children: A Systematic Review. J. Acad. Nutr. Diet. 2012, 112, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, M.H.; Haghighatdoost, F.; Surkan, P.J.; Azadbakht, L. Associations between Dietary Energy Density and Obesity: A Systematic Review and Meta-Analysis of Observational Studies. Nutrition 2016, 32, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-Processed Food Intake and Obesity: What Really Matters for Health—Processing or Nutrient Content? Curr. Obes. Rep. 2017, 6, 420–431. [Google Scholar] [CrossRef]
- Luiten, C.M.; Steenhuis, I.H.; Eyles, H.; Ni Mhurchu, C.; Waterlander, W.E. Ultra-Processed Foods Have the Worst Nutrient Profile, yet They Are the Most Available Packaged Products in a Sample of New Zealand Supermarkets. Public Health Nutr. 2016, 19, 530–538. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed Food and Chronic Noncommunicable Diseases: A Systematic Review and Meta-analysis of 43 Observational Studies. Obes. Rev. 2021, 22, 3146. [Google Scholar] [CrossRef] [PubMed]
- Martínez Steele, E.; Baraldi, L.G.; da Costa Louzada, M.L.; Moubarac, J.-C.; Mozaffarian, D.; Monteiro, C.A. Ultra-Processed Foods and Added Sugars in the US Diet: Evidence from a Nationally Representative Cross-Sectional Study. BMJ Open. 2016, 6, e009892. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global Sodium Consumption and Death from Cardiovascular Causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef] [Green Version]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary Fiber and Health Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhao, L.-G.; Wu, Q.-J.; Ma, X.; Xiang, Y.-B. Association Between Dietary Fiber and Lower Risk of All-Cause Mortality: A Meta-Analysis of Cohort Studies. Am. J. Epidemiol. 2015, 181, 83–91. [Google Scholar] [CrossRef]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-Processed Foods and the Nutrition Transition: Global, Regional and National Trends, Food Systems Transformations and Political Economy Drivers. Obes. Rev. 2020, 21, e13126. [Google Scholar] [CrossRef]
- Boyland, E.J.; Nolan, S.; Kelly, B.; Tudur-Smith, C.; Jones, A.; Halford, J.C.; Robinson, E. Advertising as a Cue to Consume: A Systematic Review and Meta-Analysis of the Effects of Acute Exposure to Unhealthy Food and Nonalcoholic Beverage Advertising on Intake in Children and Adults. Am. J. Clin. Nutr. 2016, 103, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Robinson, E.; Aveyard, P.; Daley, A.; Jolly, K.; Lewis, A.; Lycett, D.; Higgs, S. Eating Attentively: A Systematic Review and Meta-Analysis of the Effect of Food Intake Memory and Awareness on Eating. Am. J. Clin. Nutr. 2013, 97, 728–742. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.-C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA Food Classification and the Trouble with Ultra-Processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef] [Green Version]
- English, L.K.; Fearnbach, S.N.; Wilson, S.J.; Fisher, J.O.; Savage, J.S.; Rolls, B.J.; Keller, K.L. Food Portion Size and Energy Density Evoke Different Patterns of Brain Activation in Children. Am. J. Clin. Nutr. 2017, 105, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gearhardt, A.N.; Hebebrand, J. The Concept of “Food Addiction” Helps Inform the Understanding of Overeating and Obesity: YES. Am. J. Clin. Nutr. 2021, 113, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Gramza-Michałowska, A. The Effects of Ultra-Processed Food Consumption-Is There Any Action Needed? Nutrients 2020, 12, 2556. [Google Scholar] [CrossRef]
- Zhang, Y.; Venkitasamy, C.; Pan, Z.; Liu, W.; Zhao, L. Novel Umami Ingredients: Umami Peptides and Their Taste. J. Food Sci. 2017, 82, 16–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Venkitasamy, C.; Pan, Z.; Ke, H.; Guo, S.; Wu, D.; Wu, W.; Zhao, L. Potential Effects of Umami Ingredients on Human Health: Pros and Cons. Crit. Rev. Food Sci. Nutr. 2020, 60, 2294–2302. [Google Scholar] [CrossRef]
- Teo, P.S.; Tso, R.; van Dam, R.M.; Forde, C.G. Taste of Modern Diets: The Impact of Food Processing on Nutrient Sensing and Dietary Energy Intake. J. Nutr. 2022, 152, 200–210. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.-C.; Levy, R.B.; Canella, D.S.; da Costa Louzada, M.L.; Cannon, G. Household Availability of Ultra-Processed Foods and Obesity in Nineteen European Countries. Public Health Nutr. 2018, 21, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, C.; Grimes, C.; Baker, P.; Sievert, K.; Lawrence, M.A. The Drivers, Trends and Dietary Impacts of Non-Nutritive Sweeteners in the Food Supply: A Narrative Review. Nutr. Res. Rev. 2021, 34, 185–208. [Google Scholar] [CrossRef]
- Shum, B.; Georgia, S. The Effects of Non-Nutritive Sweetener Consumption in the Pediatric Populations: What We Know, What We Don’t, and What We Need to Learn. Front. Endocrinol. 2021, 12, 625415. [Google Scholar] [CrossRef]
- Cummings, J.R.; Schiestl, E.T.; Tomiyama, A.J.; Mamtora, T.; Gearhardt, A.N. Highly Processed Food Intake and Immediate and Future Emotions in Everyday Life. Appetite 2022, 169, 105868. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.S.; Del-Ponte, B.; Assunção, M.C.F.; Santos, I.S. Consumption of Ultra-Processed Foods and Body Fat during Childhood and Adolescence: A Systematic Review. Public Health Nutr. 2018, 21, 148–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coricelli, C.; Toepel, U.; Notter, M.-L.; Murray, M.M.; Rumiati, R.I. Distinct Brain Representations of Processed and Unprocessed Foods. Eur. J. Neurosci. 2019, 50, 3389–3401. [Google Scholar] [CrossRef] [PubMed]
- Gibney, M.J.; Forde, C.G.; Mullally, D.; Gibney, E.R. Ultra-Processed Foods in Human Health: A Critical Appraisal. Am. J. Clin. Nutr. 2017, 106, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Pineda, E.; Poelman, M.P.; Aaspõllu, A.; Bica, M.; Bouzas, C.; Carrano, E.; De Miguel-Etayo, P.; Djojosoeparto, S.; Blenkuš, M.G.; Graca, P.; et al. Policy Implementation and Priorities to Create Healthy Food Environments Using the Healthy Food Environment Policy Index (Food-EPI): A Pooled Level Analysis across Eleven European Countries. Lancet Reg. Health Eur. 2022, 23, 100522. [Google Scholar] [CrossRef]
- Popkin, B.M.; Barquera, S.; Corvalan, C.; Hofman, K.J.; Monteiro, C.; Ng, S.W.; Swart, E.C.; Taillie, L.S. Towards Unified and Impactful Policies to Reduce Ultra-Processed Food Consumption and Promote Healthier Eating. Lancet Diabetes Endocrinol. 2021, 9, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.; Kotov, A.; Wang, S.; Murukutla, N. “Warning: Ultra-Processed”-A Call for Warnings on Foods That Aren’t Really Foods. BMJ Glob. Health 2021, 6, e007240. [Google Scholar] [CrossRef] [PubMed]
- Dereń, K.; Dembiński, Ł.; Wyszyńska, J.; Mazur, A.; Weghuber, D.; Łuszczki, E.; Hadjipanayis, A.; Koletzko, B. Front-Of-Pack Nutrition Labelling: A Position Statement of the European Academy of Paediatrics and the European Childhood Obesity Group. Ann. Nutr. Metab. 2021, 77, 23–28. [Google Scholar] [CrossRef]
- Scharf, R.J.; DeBoer, M.D. Sugar-Sweetened Beverages and Children’s Health. Annu. Rev. Public Health 2016, 37, 273–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, A.S.; Bensignor, M.O.; Hsia, D.S.; Shoemaker, A.H.; Shih, W.; Peterson, C.; Varghese, S.T. Phentermine/Topiramate for the Treatment of Adolescent Obesity. NEJM Evid. 2022, 1, 14. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Cena, H.; Rossi, V.; Santero, S.; Bianchi, A.; Zuccotti, G. Ultra-Processed Food, Reward System and Childhood Obesity. Children 2023, 10, 804. https://doi.org/10.3390/children10050804
Calcaterra V, Cena H, Rossi V, Santero S, Bianchi A, Zuccotti G. Ultra-Processed Food, Reward System and Childhood Obesity. Children. 2023; 10(5):804. https://doi.org/10.3390/children10050804
Chicago/Turabian StyleCalcaterra, Valeria, Hellas Cena, Virginia Rossi, Sara Santero, Alice Bianchi, and Gianvincenzo Zuccotti. 2023. "Ultra-Processed Food, Reward System and Childhood Obesity" Children 10, no. 5: 804. https://doi.org/10.3390/children10050804