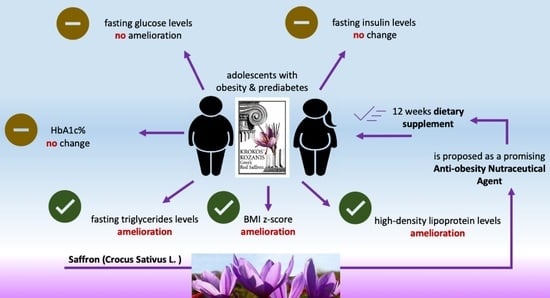
The Effect of Saffron Kozanis (Crocus sativus L.) Supplementation on Weight Management, Glycemic Markers and Lipid Profile in Adolescents with Obesity: A Double-Blinded Randomized Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria, Exclusion Criteria and Definitions
2.3. Sample Size Calculation
2.4. Anthropometric and Clinical Assessment
2.5. Biochemical Measurement
2.6. Crocus sativus L. Preparation
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants and Intervention
3.2. Demographic and Anthromopetricindices of Participants before and after Intervention
3.3. Glycemic and Lipidemic Profile before and after Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Leitzmann, M.; Freisling, H.; Bray, F.; Romieu, I.; Renehan, A.; Soerjomataram, I. Obesity and Cancer: An Update of the Global Impact. Cancer Epidemiol. 2016, 41, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kumar, P.; Srivastava, S.; Banerjee, A. Association of Anthropometric Measures of Obesity and Physical Activity with Cardio-Vascular Diseases among Older Adults: Evidence from a Cross-Sectional Survey, 2017–2018. PLoS ONE 2021, 16, e0260148. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.F.; D’Agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and Obesity as Determinants of Cardiovascular Risk. Arch. Intern. Med. 2002, 162, 1867. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Chong, M.; Le, A.; Mohammadi-Shemirani, P.; Morton, R.; Brinza, C.; Kiflen, M.; Narula, S.; Akhabir, L.; Mao, S.; et al. Surrogate Adiposity Markers and Mortality. JAMA Netw. Open 2023, 6, e2334836. [Google Scholar] [CrossRef]
- World Health Organization. Report of the Commission on Ending Childhood Obesity; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Hasani-Ranjbar, S.; Jouyandeh, Z.; Abdollahi, M. A Systematic Review of Anti-Obesity Medicinal Plants—An Update. J. Diabetes Metab. Disord. 2013, 12, 28. [Google Scholar] [CrossRef]
- Kakisis, J.D. Saffron: From Greek Mythology to Contemporary Anti-Atherosclerotic Medicine. Atherosclerosis 2018, 268, 193–195. [Google Scholar] [CrossRef]
- European Commission. Krokos Kozanis PDO; European Commission: Brussels, Belgium, 1997. [Google Scholar]
- Hatziagapiou, K.; Lambrou, G.I. The Protective Role of Crocus sativus L. (Saffron) Against Ischemia- Reperfusion Injury, Hyperlipidemia and Atherosclerosis: Nature Opposing Cardiovascular Diseases. Curr. Cardiol. Rev. 2018, 14, 272–289. [Google Scholar] [CrossRef]
- Mashmoul, M.; Azlan, A.; Khaza’Ai, H.; Yusof, B.N.M.; Noor, S.M. Saffron: A Natural Potent Antioxidant as a Promising Anti-Obesity Drug. Antioxidants 2013, 2, 293–308. [Google Scholar] [CrossRef]
- Ahmadikhatir, S.; Ostadrahimi, A.; Safaiyan, A.; Ahmadikhatir, S.; Farrin, N. Saffron (Crocus sativus L.) Supplements Improve Quality of Life and Appetite in Atherosclerosis Patients: A Randomized Clinical Trial. J. Res. Med. Sci. 2022, 27, 30. [Google Scholar] [CrossRef]
- Kermani, T.; Kazemi, T.; Molki, S.; Ilkhani, K.; Sharifzadeh, G.; Rajabi, O. The Efficacy of Crocin of Saffron (Crocus sativus L.) on the Components of Metabolic Syndrome: A Randomized Controlled Clinical Trial. J. Res. Pharm. Pract. 2017, 6, 228. [Google Scholar] [CrossRef]
- Milajerdi, A.; Jazayeri, S.; Hashemzadeh, N.; Shirzadi, E.; Derakhshan, Z.; Djazayeri, A.; Akhondzadeh, S. The Effect of Saffron (Crocus sativus L.) Hydroalcoholic Extract on Metabolic Control in Type 2 Diabetes Mellitus: A Triple-Blinded Randomized Clinical Trial. J. Res. Med. Sci. 2018, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, A.; Khajehlandi, M.; Siahkuhian, M.; Akbarnejad, A.; Khoramipour, K.; Suzuki, K. Effect of 8 Weeks Aerobic Training and Saffron Supplementation on Inflammation and Metabolism in Middle-Aged Obese Women with Type 2 Diabetes Mellitus. Sports 2022, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Nazari, E.; Nadjarzadeh, A.; Masoumi, R.; Marzban, A.; Mohajeri, S.A.; Ramezani-Jolfaie, N.; Salehi-Abargouei, A. Effect of Saffron (Crocus sativus L.) on Lipid Profile, Glycemic Indices and Antioxidant Status among Overweight/Obese Prediabetic Individuals: A Double-Blinded, Randomized Controlled Trial. Clin. Nutr. ESPEN 2019, 34, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Tajaddini, A.; Roshanravan, N.; Mobasseri, M.; Aeinehchi, A.; Sefid-Mooye Azar, P.; Hadi, A.; Ostadrahimi, A. Saffron Improves Life and Sleep Quality, Glycaemic Status, Lipid Profile and Liver Function in Diabetic Patients: A Double-Blind, Placebo-Controlled, Randomised Clinical Trial. Int. J. Clin. Pract. 2021, 75, e14334. [Google Scholar] [CrossRef]
- Mobasseri, M.; Ostadrahimi, A.; Tajaddini, A.; Asghari, S.; Barati, M.; Akbarzadeh, M.; Nikpayam, O.; Houshyar, J.; Roshanravan, N.; Alamdari, N.M. Effects of Saffron Supplementation on Glycemia and Inflammation in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind, Placebo-Controlled Clinical Trial Study. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 527–534. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Aryaeian, N.; Pahlavani, N.; Abbasi, D.; Hosseini, A.F.; Fallah, S.; Moradi, N.; Heydari, I. The Effect of Saffron (Crocus sativus L.) Supplementation on Blood Pressure, and Renal and Liver Function in Patients with Type 2 Diabetes Mellitus: A Double-Blinded, Randomized Clinical Trial. Avicenna J. Phytomed. 2019, 9, 322–333. [Google Scholar]
- Moravej Aleali, A.; Amani, R.; Shahbazian, H.; Namjooyan, F.; Latifi, S.M.; Cheraghian, B. The Effect of Hydroalcoholic Saffron (Crocus sativus L.) Extract on Fasting Plasma Glucose, HbA1c, Lipid Profile, Liver, and Renal Function Tests in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Phytother. Res. 2019, 33, 1648–1657. [Google Scholar] [CrossRef]
- Hooshmand Moghadam, B.; Rashidlamir, A.; Attarzadeh Hosseini, S.R.; Gaeini, A.A.; Kaviani, M. The Effects of Saffron (Crocus sativus L.) in Conjunction with Concurrent Training on Body Composition, Glycaemic Status, and Inflammatory Markers in Obese Men with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Br. J. Clin. Pharmacol. 2022, 88, 3256–3271. [Google Scholar] [CrossRef]
- Tahmasbi, F.; Araj-Khodaei, M.; Mahmoodpoor, A.; Sanaie, S. Effects of Saffron (Crocus sativus L.) on Anthropometric and Cardiometabolic Indices in Overweight and Obese Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phytother. Res. 2022, 36, 3394–3414. [Google Scholar] [CrossRef]
- Asbaghi, O.; Soltani, S.; Norouzi, N.; Milajerdi, A.; Choobkar, S.; Asemi, Z. The Effect of Saffron Supplementation on Blood Glucose and Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2019, 47, 102158. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. 2000. Available online: www.wma.net (accessed on 1 May 2020).
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity—Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Fontecilla, H.; Moyano-Ramírez, E.; Méndez-González, O.; Rodrigo-Yanguas, M.; Martin-Moratinos, M.; Bella-Fernández, M. Effectivity of Saffron Extract (Saffr’Activ) on Treatment for Children and Adolescents with Attention Deficit/Hyperactivity Disorder (ADHD): A Clinical Effectivity Study. Nutrients 2022, 14, 4046. [Google Scholar] [CrossRef] [PubMed]
- Baziar, S.; Aqamolaei, A.; Khadem, E.; Mortazavi, S.H.; Naderi, S.; Sahebolzamani, E.; Mortezaei, A.; Jalilevand, S.; Mohammadi, M.R.; Shahmirzadi, M.; et al. Crocus sativus L. Versus Methylphenidate in Treatment of Children with Attention-Deficit/Hyperactivity Disorder: A Randomized, Double-Blind Pilot Study. J. Child Adolesc. Psychopharmacol. 2019, 29, 205–212. [Google Scholar] [CrossRef]
- Boston Children’s Hospital BMI z-Score System. Available online: https://zscore.chboston.org (accessed on 20 August 2023).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and ?-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Garza-Juárez, A.; Pérez-Carrillo, E.; Arredondo-Espinoza, E.U.; Islas, J.F.; Benítez-Chao, D.F.; Escamilla-García, E. Nutraceuticals and Their Contribution to Preventing Noncommunicable Diseases. Foods 2023, 12, 3262. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P. Gut Microbiome and Type 2 Diabetes: Where We Are and Where to Go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef]
- Zhu, T.; Goodarzi, M.O. Metabolites Linking the Gut Microbiome with Risk for Type 2 Diabetes. Curr. Nutr. Rep. 2020, 9, 83–93. [Google Scholar] [CrossRef]
- Razavi, B.M.; Hosseinzadeh, H. Saffron: A Promising Natural Medicine in the Treatment of Metabolic Syndrome. J. Sci. Food Agric. 2017, 97, 1679–1685. [Google Scholar] [CrossRef]
- Shahinfar, H.; Jayedi, A.; Torabynasab, K.; Payandeh, N.; Martami, F.; Moosavi, H.; Bazshahi, E.; Shab-Bidar, S. Comparative Effects of Nutraceuticals on Body Weight in Adults with Overweight or Obesity: A Systematic Review and Network Meta-Analysis of 111 Randomized Clinical Trials. Pharmacol. Res. 2023, 196, 106944. [Google Scholar] [CrossRef] [PubMed]
- Pourmasoumi, M.; Hadi, A.; Najafgholizadeh, A.; Kafeshani, M.; Sahebkar, A. Clinical Evidence on the Effects of Saffron (Crocus sativus L.) on Cardiovascular Risk Factors: A Systematic Review Meta-Analysis. Pharmacol. Res. 2019, 139, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, J.; Manzari, N.; Thompson, J.; Clark, C.C.T.; Villanueva, G.; Varkaneh, H.K.; Mirmiran, P. The Effect of Saffron on Weight and Lipid Profile: A Systematic Review, Meta-analysis, and Dose–Response of Randomized Clinical Trials. Phytother. Res. 2019, 33, 2244–2255. [Google Scholar] [CrossRef] [PubMed]
- Sohaei, S.; Hadi, A.; Karimi, E.; Arab, A. Saffron Supplementation Effects on Glycemic Indices: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Int. J. Food Prop. 2020, 23, 1386–1401. [Google Scholar] [CrossRef]
- Rahmani, J.; Bazmi, E.; Clark, C.; Hashemi Nazari, S.S. The Effect of Saffron Supplementation on Waist Circumference, HA1C, and Glucose Metabolism: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Complement. Ther. Med. 2020, 49, 102298. [Google Scholar] [CrossRef]
- Naserizadeh, S.K.; Taherifard, M.H.; Shekari, M.; Mesrkanlou, H.A.; Asbaghi, O.; Nazarian, B.; Khosroshahi, M.Z.; Heydarpour, F. The Effect of Crocin Supplementation on Lipid Concentrations and Fasting Blood Glucose: A Systematic Review and Meta-Analysis and Meta-Regression of Randomized Controlled Trials. Complement. Ther. Med. 2020, 52, 102500. [Google Scholar] [CrossRef]
- Roshanravan, B.; Samarghandian, S.; Ashrafizadeh, M.; Amirabadizadeh, A.; Saeedi, F.; Farkhondeh, T. Metabolic Impact of Saffron and Crocin: An Updated Systematic and Meta-Analysis of Randomised Clinical Trials. Arch. Physiol. Biochem. 2022, 128, 666–678. [Google Scholar] [CrossRef]
- Yan, Y.; Gong, Y.; Jiang, M.; Gao, Y.; Guo, S.; Huo, J.; Zhao, Z.; Li, C. Utilization of Glucagon-like Peptide-1 Receptor Agonists in Children and Adolescents in China: A Real-World Study. Front. Endocrinol. 2023, 14, 1170127. [Google Scholar] [CrossRef]
- Lim, F.; Bellows, B.K.; Tan, S.X.; Aziz, Z.; Woo Baidal, J.A.; Kelly, A.S.; Hur, C. Cost-Effectiveness of Pharmacotherapy for the Treatment of Obesity in Adolescents. JAMA Netw. Open 2023, 6, e2329178. [Google Scholar] [CrossRef]

| Variable | Study Groups | p-Value | ||
|---|---|---|---|---|
| Crocus Sativus (n = 25) | Metformin (n = 25) | Placebo (n = 24) | ||
| Gender (male/female) | 12/13 | 11/13 | 11/13 | 0.841 |
| Age (years) | 12.32 ± 1.43 | 12.59 ± 1.44 | 12.27 ± 1.87 | 0.626 |
| Height (cm) | 159.60 ± 10.94 | 159.73 ± 10.04 | 156.93 ± 14.97 | 0.386 |
| Height z-score | 1.00 ± 1.37 | 0.89 ± 0.80 | 0.86 ± 1.72 | 0.802 |
| Weight (kg) | 73.43 ± 15.28 | 76.31 ± 12.27 | 74.71 ± 24.7 | 0.431 |
| Weight z-score | 2.16 ± 0.49 | 2.23 ± 0.43 | 2.18 ± 0.73 | 0.545 |
| BMI | 28.62 ± 3.47 | 29.78 ± 3.39 | 29.72 ± 5.83 | 0.422 |
| BMI z-score | 2.04 ± 0.31 | 2.07 ± 0.30 | 2.07 ± 0.38 | 0.802 |
| WC (cm) | 95.27 ± 9.07 | 96.38 ± 7.12 | 95.05 ± 4.13 | 0.561 |
| SBP (mmHg) | 116.21 ± 9.73 | 119.44 ± 10.46 | 112.96 ± 10.43 | 0.128 |
| DBP (mmHg) | 69.00 ± 10.81 | 73.72 ± 9.51 | 72.58 ± 9.42 | 0.237 |
| Variable | Study Groups | p-Value * | |||
|---|---|---|---|---|---|
| Crocus Sativus (n = 25) | Metformin (n = 25) | Placebo (n = 24) | |||
| Height (cm) | Before | 159.60 ± 10.94 | 159.73 ± 10.04 | 156.93 ± 14.97 | |
| After | 161.07 ± 11.04 | 161.00 ± 10.40 | 158.54 ± 14.62 | 0.746 | |
| p-value ** | <0.001 | <0.001 | <0.001 | ||
| Height z-score | Before | 1.00 ± 1.37 | 0.89 ± 0.80 | 0.86 ± 1.72 | |
| After | 1.00 ± 1.37 | 0.89 ± 0.86 | 0.89 ± 1.73 | 0.951 | |
| p-value ** | 0.802 | 0.967 | 0.131 | ||
| Weight (kg) | Before | 73.43 ± 15.28 | 76.31 ± 12.27 | 74.71 ± 24.7 | |
| After | 72.49 ± 15.59 | 72.12 ± 12.89 | 76.02 ± 23.78 | 0.374 | |
| p-value ** | 0.092 | <0.001 | 0.008 | ||
| Weight z-score | Before | 2.16 ± 0.49 | 2.23 ± 0.43 | 2.18 ± 0.73 | |
| After | 2.02 ± 0.56 | 1.93 ± 0.55 | 2.24 ± 0.76 | 0.224 | |
| p-value ** | <0.001 | <0.001 | 0.269 | ||
| BMI | Before | 28.62 ± 3.47 | 29.78 ± 3.39 | 29.72 ± 5.83 | |
| After | 27.71 ± 3.55 | 27.64 ± 3.26 | 29.68 ± 5.43 | 0.076 | |
| p-value ** | <0.001 | <0.001 | 0.781 | ||
| BMI z-score | Before | 2.04 ± 0.31 | 2.07 ± 0.30 | 2.07 ± 0.38 | |
| After | 1.90 ± 0.33 | 1.81 ± 0.35 | 2.06 ± 0.37 | 0.052 | |
| p-value ** | <0.001 | <0.001 | 0.496 | ||
| WC (cm) | Before | 95.27 ± 9.07 | 96.38 ± 7.12 | 95.05 ± 4.13 | |
| After | 92.75 ± 9.27 | 92.36 ± 8.15 | 95.29 ± 13.45 | 0.499 | |
| p-value ** | 0.042 | <0.001 | 0.710 | ||
| SBP (mmHg) | Before | 116.21 ± 9.73 | 119.44 ± 10.46 | 112.96 ± 10.43 | |
| After | 115.04 ± 8.77 | 117.52 ± 8.83 | 113.91 ± 10.71 | 0.605 | |
| p-value ** | 0.553 | 0.416 | 0.729 | ||
| DBM (mmHg) | Before | 69.00 ± 10.81 | 73.72 ± 9.51 | 72.58 ± 9.42 | |
| After | 69.58 ± 7.59 | 68.28 ± 7.23 | 70.62 ± 8.36 | 0.398 | |
| p-value ** | 0.812 | 0.037 | 0.454 |
| Variable | Group | Dif Mean ± SD | p-Value * | p-Value ** | p-Value *** |
|---|---|---|---|---|---|
| Weight (kg) | Crocus sativus | −1.02 ± 2.91 | |||
| Metformin | −4.18 ± 4.50 | <0.001 | 0.006 | 0.001 | |
| Placebo | +1.30 ± 2.23 | ||||
| Weight z-score | Crocus sativus | −0.14 ± 0.14 | |||
| Metformin | −0.39 ± 0.22 | <0.001 | 0.010 | <0.001 | |
| Placebo | −0.05 ± 0.26 | ||||
| BMI | Crocus sativus | −0.95 ± 1.11 | |||
| Metformin | −2.15 ± 1.91 | <0.001 | 0.013 | 0.003 | |
| Placebo | −0.04 ± 0.72 | ||||
| BMI z-score | Crocus sativus | −0.14 ± 0.13 | |||
| Metformin | −0.26 ± 0.18 | <0.001 | 0.016 | <0.001 | |
| Placebo | −0.01 ± 0.08 | ||||
| WC (cm) | Crocus sativus | −2.66 ± 6.19 | |||
| Metformin | −4.01 ± 4.88 | 0.004 | 0.579 | 0.027 | |
| Placebo | +0.24 ± 3.19 | ||||
| SBP (mmHg) | Crocus sativus | −1.12 ± 9.30 | |||
| Metformin | −1.92 ± 11.60 | 0.856 | 0.683 | 0.779 | |
| Placebo | +0.95 ± 13.37 | ||||
| DBM (mmHg) | Crocus sativus | −4.23 ± 11.61 | |||
| Metformin | −5.44 ± 12.33 | 0.260 | 0.109 | 0.496 | |
| Placebo | −1.95 ± 12.59 |
| Variable | Study Groups | p-Value * | |||
|---|---|---|---|---|---|
| Crocus Sativus (n = 25) | Metformin (n = 25) | Placebo (n = 24) | |||
| Fasting Glucose (mg/dL) | Before | 103.04 ± 9.28 | 103.80 ± 12.41 | 103.58 ± 6.16 | 0.966 |
| After | 99.96 ± 9.48 | 95.80 ± 12.16 | 98.66 ± 10.61 | 0.471 | |
| p-value ** | 0.218 | 0.036 | 0.083 | ||
| Fasting Insulin (μIU/mL) | Before | 22.84 ± 8.78 | 22.91 ± 6.51 | 22.30 ± 7.24 | 0.955 |
| After | 19.96 ± 10.59 | 17.18 ± 7.83 | 23.35 ± 9.05 | 0.065 | |
| p-value ** | 0.192 | 0.004 | 0.440 | ||
| HbA1c% | Before | 5.33 ± 0.28 | 5.30 ± 0.19 | 5.28 ± 0.26 | 0.738 |
| After | 5.25 ± 0.33 | 5.27 ± 0.19 | 5.19 ± 0.28 | 0.496 | |
| p-value ** | 0.183 | 0.513 | 0.100 | ||
| HOMA-IR | Before | 5.83 ± 2.41 | 5.91 ± 2.02 | 5.71 ± 1.92 | 0.951 |
| After | 5.02 ± 2.85 | 4.07 ± 1.91 | 5.83 ± 2.92 | 0.063 | |
| p-value ** | 0.184 | 0.001 | 0.804 | ||
| Fasting Cholesterol (mg/dL) | Before | 157.08 ± 23.85 | 154.04 ± 26.97 | 150.46 ± 20.80 | 0.633 |
| After | 154.04 ± 22.78 | 142.60 ± 33.40 | 148.37 ± 18.96 | 0.281 | |
| p-value ** | 0.415 | 0.003 | 0.288 | ||
| Fasting HDL (mg/dL) | Before | 45.62 ± 9.52 | 45.84 ± 13.45 | 46.79 ± 8.22 | 0.931 |
| After | 48.29 ± 8.37 | 47.76 ± 11.87 | 46.66 ± 10.99 | 0.820 | |
| p-value ** | 0.023 | 0.103 | 0.906 | ||
| Fasting LDL (mg/dL) | Before | 86.44 ± 21.56 | 84.20 ± 21.52 | 82.58 ± 18.40 | 0.774 |
| After | 86.72 ± 20.02 | 74.28 ± 27.20 | 80.91 ± 16.95 | 0.116 | |
| p-value ** | 0.931 | 0.001 | 0.271 | ||
| Fasting Triglycerides (mg/dl) | Before | 100.08 ± 36.65 | 98.24 ± 43.41 | 97.12 ± 11.43 | 0.933 |
| After | 81.20 ± 33.71 | 96.08 ± 50.5 | 101.25 ± 24.70 | 0.178 | |
| p-value ** | <0.001 | 0.827 | 0.293 | ||
| Uric Acid (mg/dL) | Before | 4.86 ± 1.08 | 5.13 ± 1.07 | 5.03 ± 1.11 | 0.545 |
| After | 4.96 ± 1.04 | 4.94 ± 1.10 | 5.15 ± 0.95 | 0.722 | |
| p-value ** | 0.368 | 0.067 | 0.341 | ||
| Urea (mg/dL) | Before | 23.12 ± 7.29 | 25.60 ± 7.39 | 25.95 ± 4.89 | 0.222 |
| After | 25.52 ± 5.80 | 24.12 ± 5.08 | 24.82 ± 5.82 | 0.864 | |
| p-value ** | 0.066 | 0.274 | 0.417 | ||
| Creatinine (mg/dL) | Before | 0.56 ± 0.09 | 0.59 ± 0.11 | 0.56 ± 0.11 | 0.504 |
| After | 0.59 ± 0.13 | 0.61 ± 0.10 | 0.55 ± 0.11 | 0.276 | |
| p-value ** | 0.100 | 0.369 | 0.451 |
| Variable | Group | Dif Mean ± SD | p-Value * | p-Value ** | p-Value *** |
|---|---|---|---|---|---|
| Fasting Glucose (mg/dL) | Crocus sativus | −3.08 ± 12.17 | |||
| Metformin | −8.00 ± 18.05 | 0.159 | 0.082 | 0.307 | |
| Placebo | −4.92 ± 13.28 | ||||
| Fasting Insulin (μIU/mL) | Crocus sativus | −2.87 ± 10.72 | |||
| Metformin | −5.74 ± 8.96 | 0.005 | 0.256 | 0.039 | |
| Placebo | +1.05 ± 6.55 | ||||
| HbA1c% | Crocus sativus | −0.08 ± 0.23 | |||
| Metformin | −0.02 ± 0.18 | 0.643 | 0.330 | 0.791 | |
| Placebo | −0.08 ± 0.23 | ||||
| HOMA-IR | Crocus sativus | −0.80 ± 2.95 | |||
| Metformin | −1.84 ± 2.33 | 0.024 | 0.233 | 0.215 | |
| Placebo | +0.13 ± 2.50 | ||||
| Fasting Cholesterol (mg/dL) | Crocus sativus | −2.92 ± 17.57 | |||
| Metformin | −11.44 ± 17.44 | 0.057 | 0.054 | 0.347 | |
| Placebo | +2.08 ± 9.38 | ||||
| Fasting HDL (mg/dL) | Crocus sativus | +2.56 ± 5.26 | |||
| Metformin | +1.92 ± 5.67 | 0.013 | 0.598 | 0.008 | |
| Placebo | −0.13 ± 5.12 | ||||
| Fasting LDL (mg/dL) | Crocus sativus | +0.28 ± 15.92 | |||
| Metformin | −9.92 ± 12.35 | 0.013 | 0.012 | 0.616 | |
| Placebo | −1.66 ± 7.23 | ||||
| Fasting Triglycerides (mg/dL) | Crocus sativus | −18.88 ± 22.32 | |||
| Metformin | −2.16 ± 48.84 | 0.004 | 0.086 | <0.001 | |
| Placebo | +4.12 ± 18.78 | ||||
| Uric acid (mg/dL) | Crocus sativus | −0.08 ± 0.44 | |||
| Metformin | −0.19 ± 0.49 | 0.141 | 0.117 | 0.763 | |
| Placebo | +0.12 ± 0.60 | ||||
| Urea (mg/dL) | Crocus sativus | +2.12 ± 5.39 | |||
| Metformin | −1.48 ± 6.60 | 0.158 | 0.082 | 0.120 | |
| Placebo | −1.08 ± 6.41 | ||||
| Creatinine (mg/dL) | Crocus sativus | −0.02 ± 0.07 | |||
| Metformin | −0.01 ± 0.06 | 0.150 | 0.695 | 0.075 | |
| Placebo | −0.01 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotanidou, E.P.; Tsinopoulou, V.R.; Giza, S.; Ntouma, S.; Angeli, C.; Chatziandreou, M.; Tsopelas, K.; Tseti, I.; Galli-Tsinopoulou, A. The Effect of Saffron Kozanis (Crocus sativus L.) Supplementation on Weight Management, Glycemic Markers and Lipid Profile in Adolescents with Obesity: A Double-Blinded Randomized Placebo-Controlled Trial. Children 2023, 10, 1814. https://doi.org/10.3390/children10111814
Kotanidou EP, Tsinopoulou VR, Giza S, Ntouma S, Angeli C, Chatziandreou M, Tsopelas K, Tseti I, Galli-Tsinopoulou A. The Effect of Saffron Kozanis (Crocus sativus L.) Supplementation on Weight Management, Glycemic Markers and Lipid Profile in Adolescents with Obesity: A Double-Blinded Randomized Placebo-Controlled Trial. Children. 2023; 10(11):1814. https://doi.org/10.3390/children10111814
Chicago/Turabian StyleKotanidou, Eleni P., Vasiliki Rengina Tsinopoulou, Styliani Giza, Stergianna Ntouma, Chrysanthi Angeli, Michail Chatziandreou, Konstantinos Tsopelas, Ioulia Tseti, and Assimina Galli-Tsinopoulou. 2023. "The Effect of Saffron Kozanis (Crocus sativus L.) Supplementation on Weight Management, Glycemic Markers and Lipid Profile in Adolescents with Obesity: A Double-Blinded Randomized Placebo-Controlled Trial" Children 10, no. 11: 1814. https://doi.org/10.3390/children10111814