Physical Image Quality Metrics for the Characterization of X-ray Systems Used in Fluoroscopy-Guided Pediatric Cardiac Interventional Procedures: A Systematic Review
Abstract
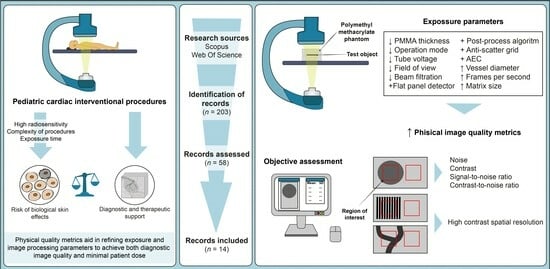
:1. Introduction
- ▪
- RQ 1: What are the primary physical image quality metrics commonly utilized for characterizing X-ray systems employed in fluoroscopy-guided pediatric interventional cardiac procedures, and what are the prevalent methods employed to measure these metrics?
- ▪
- RQ 2: What factors have been examined in the literature for their impact on physical image quality metrics in characterizing X-ray systems used in fluoroscopy-guided pediatric interventional cardiac procedures?
2. Materials and Methods
3. Results
3.1. Physical Image Quality Assessment
3.2. Equations Utilized for Physical Image Quality Metrics Calculation
3.2.1. N Objective Estimation
3.2.2. SNR Objective Estimation
3.2.3. C Objective Estimation
3.2.4. CNR Objective Estimation
3.2.5. HCSR Objective Estimation
3.3. Factors Influencing Image Quality
3.4. Assessment of Bias Risk
4. Discussion
4.1. What Are the Primary Physical Image Quality Metrics Commonly Utilized for Characterizing X-ray Systems Employed in Fluoroscopy-Guided Pediatric Interventional Cardiac Procedures, and What Are the Prevalent Methods Employed to Measure These Metrics?
4.1.1. N
4.1.2. SNR
4.1.3. C
4.1.4. CNR
4.1.5. HCSR
4.2. What Factors Have Been Examined in the Literature for Their Impact on Physical Image Quality Metrics in Characterizing X-ray Systems Used in Fluoroscopy-Guided Pediatric Interventional Cardiac Procedures?
4.2.1. Polymethyl Methacrylate (PMMA) Thickness
4.2.2. Operation Mode
4.2.3. Anti-Scatter Grid
4.2.4. Tube Voltage
4.2.5. Field of View (FOV)
4.2.6. Vessel Diameter
4.2.7. Frames Per Second
4.2.8. Contrast Mode
4.2.9. Fluoroscopic System
4.2.10. Matrix Size
4.2.11. Filter Thickness
4.2.12. AEC Program
4.3. Final Remarks
4.4. Study Limitations
4.5. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ubeda, C.; Salazar, L.; Retana, V.; Santos, F.; Salvador, L.; Sáenz, C.; Quesada, C.; Gavarrete, M.; Picado, M.; Arce, L. Characterization and Experimental Measurements of Scatter Dose at Cardiologist’s Eyes during Paediatric Interventional Cardiology Procedures in Costa Rica. Radiat. Prot. Dosim. 2017, 176, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Miranda, P.; Dalmazzo, D. Evolution of Radiation Dose and Image Quality in a Pediatric Interventional Cardiology System [Dosis de Radiación y Calidad de Imagen En Un Equipo de Cardiología Intervencionista Pediátrico]. Interciencia 2014, 39, 518–523. [Google Scholar]
- Azizmohammadi, F.; Navarro Castellanos, I.; Miró, J.; Segars, P.; Samei, E.; Duong, L. Generative Learning Approach for Radiation Dose Reduction in X-ray Guided Cardiac Interventions. Med. Phys. 2022, 49, 4071–4081. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Vano, E.; Gonzalez, L.; Miranda, P. Influence of the Antiscatter Grid on Dose and Image Quality in Pediatric Interventional Cardiology X-ray Systems. Catheter. Cardiovasc. Interv. 2013, 82, 51–57. [Google Scholar] [CrossRef]
- Lubis, L.E.; Bayuadi, I.; Pawiro, S.A.; Ng, K.-H.; Bosmans, H.; Soejoko, D.S. Optimization of Dose and Image Quality of Paediatric Cardiac Catheterization Procedure. Phys. Medica 2015, 31, 659–668. [Google Scholar] [CrossRef]
- Vañó, E.; Ubeda, C.; Martinez, L.C.; Leyton, F.; Miranda, P. Paediatric Interventional Cardiology: Flat Detector versus Image Intensifier Using a Test Object. Phys. Med. Biol. 2010, 55, 7287–7297. [Google Scholar] [CrossRef]
- Onnasch, D.G.W.; Schemm, A.; Kramer, H.-H. Optimization of Radiographic Parameters for Paediatric Cardiac Angiography. Br. J. Radiol. 2004, 77, 479–487. [Google Scholar] [CrossRef]
- Ubeda, C.; Vano, E.; Miranda, P.; Leyton, F.; Martinez, L.C.; Oyarzun, C. Radiation Dose and Image Quality for Paediatric Interventional Cardiology Systems. A National Survey in Chile. Radiat. Prot. Dosim. 2011, 147, 429–438. [Google Scholar] [CrossRef]
- de las Heras, H.; Torres, R.; Fernández-Soto, J.M.; Vañó, E. Objective Criteria for Acceptability and Constancy Tests of Digital Subtraction Angiography. Phys. Medica 2016, 32, 272–276. [Google Scholar] [CrossRef]
- Gislason, A.J.; Davies, A.G.; Cowen, A.R. Dose Optimization in Pediatric Cardiac X-ray Imaging. Med. Phys. 2010, 37, 5258–5269. [Google Scholar] [CrossRef]
- Vano, E.; Ubeda, C.; Leyton, F.; Miranda, P. Radiation Dose and Image Quality for Paediatric Interventional Cardiology. Phys. Med. Biol. 2008, 53, 4049. [Google Scholar] [CrossRef] [PubMed]
- Bor, D.; Birgul, O.; Onal, U.; Olgar, T. Investigation of Grid Performance Using Simple Image Quality Tests. J. Med. Phys. 2016, 41, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Corredoira, E.; Vañó, E.; Alejo, L.; Ubeda, C.; Gutiérrez-Larraya, F.; Garayoa, J. Biplane Interventional Pediatric System with Cone-Beam CT: Dose and Image Quality Characterization for the Default Protocols. J. Appl. Clin. Med. Phys. 2016, 17, 357–376. [Google Scholar] [CrossRef]
- Vassileva, J.; Vano, E.; Ubeda, C.; Rehani, M.; Zotova, R. Impact of the X-ray System Setting on Patient Dose and Image Quality; a Case Study with Two Interventional Cardiology Systems. Radiat. Prot. Dosim. 2013, 155, 329–334. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Chapter 3: Systematic Reviews of Effectiveness. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; Joanna Brggs Institute: Adelaide, Australia, 2020. [Google Scholar]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Chapter 3: Systematic Reviews of Effectiveness. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; The Joanna Briggs Institute: Adelaide, Australia, 2017. [Google Scholar]
- Kordolaimi, S.D.; Salvara, A.L.N.; Antonakos, I.; Tsalafoutas, I.A.; Broutzos, E.; Efstathopoulos, E.P. Comparative Performance Evaluation of a Flat Detector and an Image Intensifier Angiographic System Both Used for Interventional Cardiology Procedures in Adult and Pediatric Patients. Phys. Medica 2013, 29, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Vano, E.; Ubeda, C.; Geiger, B.; Martinez, L.C.; Balter, S. Influence of Image Metrics When Assessing Image Quality from a Test Object in Cardiac X-ray Systems. J. Digit. Imaging 2010, 24, 331–338. [Google Scholar] [CrossRef]
- Konstantinidis, A. Physical Parameters of Image Quality. In Comprehensive Biomedical Physics; Elsevier: Amsterdam, The Netherlands, 2014; pp. 49–63. [Google Scholar] [CrossRef]
- Williams, M.B.; Krupinski, E.A.; Strauss, K.J.; Breeden, W.K.; Rzeszotarski, M.S.; Applegate, K.; Wyatt, M.; Bjork, S.; Seibert, J.A. Digital Radiography Image Quality: Image Acquisition. J. Am. Coll. Radiol. 2007, 4, 371–388. [Google Scholar] [CrossRef]
- Morin, R.; Mahesh, M. Role of Noise in Medical Imaging. J. Am. Coll. Radiol. 2018, 15, 1309. [Google Scholar] [CrossRef]
- Lamers, L.J.; Morray, B.H.; Nugent, A.; Speidel, M.; Suntharos, P.; Prieto, L. Multicenter Assessment of Radiation Exposure during Pediatric Cardiac Catheterizations Using a Novel Imaging System. J. Interv. Cardiol. 2019, 2019, 7639754. [Google Scholar] [CrossRef] [PubMed]
- Huda, W.; Sajewicz, A.M.; Ogden, K.M.; Dance, D.R. Experimental Investigation of the Dose and Image Quality Characteristics of a Digital Mammography Imaging System. Med. Phys. 2003, 30, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Nocetti, D.; Ubeda, C.; Calcagno, S.; Acevedo, J.; Pardo, D. Comparison of Image Quality among Three X-ray Systems for Chest Radiography: First Step in Optimisation. Radiat. Prot. Dosim. 2015, 165, 386–391. [Google Scholar] [CrossRef]
- Ekpo, E.U.; Hoban, A.C.; McEntee, M.F. Optimisation of Direct Digital Chest Radiography Using Cu Filtration. Radiography 2014, 20, 346–350. [Google Scholar] [CrossRef]
- Verdun, F.R.; Racine, D.; Ott, J.G.; Tapiovaara, M.J.; Toroi, P.; Bochud, F.O.; Veldkamp, W.J.H.; Schegerer, A.; Bouwman, R.W.; Hernandez-Giron, I.; et al. Image Quality in CT: From Physical Measurements to Model Observers. Phys. Medica 2015, 31, 823–843. [Google Scholar] [CrossRef] [PubMed]
- TOR 18FG—Leeds Test Objects. Available online: https://leedstestobjects.com/index.php/phantom/tor-18fg/ (accessed on 10 July 2023).
- Doyle, P.; Martin, C.J.; Gentle, D. Application of Contrast-to-Noise Ratio in Optimizing Beam Quality for Digital Chest Radiography: Comparison of Experimental Measurements and Theoretical Simulations. Phys. Med. Biol. 2006, 51, 2953. [Google Scholar] [CrossRef]
- Seeram, E. Dose Optimization in Digital Radiography. In Digital Radiography; Springer: Singapore, 2019; pp. 213–227. [Google Scholar] [CrossRef]
- Strauss, K.J. Pediatric Interventional Radiography Equipment: Safety Considerations. Pediatr. Radiol. 2006, 36, 126–135. [Google Scholar] [CrossRef]
- IEC 61223-3-3:1996|IEC Webstore. Available online: https://webstore.iec.ch/publication/19374 (accessed on 20 July 2023).
- Livingstone, R.S.; Chase, D.; Varghese, A.; George, P.V.; George, O.K. Transition from Image Intensifier to Flat Panel Detector in Interventional Cardiology: Impact of Radiation Dose. J. Med. Phys. 2015, 40, 24–28. [Google Scholar] [CrossRef]
- Konst, B.; Nøtthellen, J.; Bilet, E.; Båth, M. Radiographic and Fluoroscopic X-ray Systems: Quality Control of the X-ray Tube and Automatic Exposure Control Using Theoretical Spectra to Determine Air Kerma and Dose to a Homogenous Phantom. J. Appl. Clin. Med. Phys. 2021, 22, 204–218. [Google Scholar] [CrossRef]


| First Author (Reference) | Image Quality Metric | Test Object | Software | No. Images Analyzed (Frames) | Matrix Size (Bit Depth) |
|---|---|---|---|---|---|
| Ubeda et al. [1] | SNR, HCSR | TOR 18FG | Osiris 4.19 | 3 (10, 12, 15) | 512 × 512 (8 bits) |
| Bor et al. [12] | SNR, C, HCSR | LCD4 Hüttner type 18 | N.R. | N.R. | N.R. |
| De las Heras et al. [9] | CNR | IEC type B | N.R. | N.R. | N.R. |
| Corredoira et al. [13] | SNR, HCSR | TOR 18FG | ImageJ 1.48r | 3 (5, 8, 10) | 1024 × 1024 (12 bits) |
| Lubis et al. [5] | SNR | In-house | ImageJ | 1 (random) | N.R. |
| Ubeda et al. [2] | SNR | TOR 18FG | Osiris 4.18 | 3 (10, 12, 15) | 1024 × 1024 512 × 512 (8 bits) |
| Ubeda et al. [4] | SNR, HCSR | TOR 18FG | Osiris 4.18 | 3 (10, 12, 15) | 512 × 512 (8 bits) |
| Kordolaimi et al. [19] | SNR | 5 mm-thick aluminum plate; TOR 18FG | N.R. | N.R. | N.R. |
| Ubeda et al. [8] | SNR, HCSR | TOR 18FG | Osiris 4.18 | 3 (10, 12, 15) | 512 × 512 (8 bits) |
| Vañó et al. [20] | SNR, HCSR | TOR 18FG | Osiris 4.18 | 3 (10, 12, 15) | 1024 × 1024 (12 bits) 512 × 512 (8 bits) |
| Vañó et al. [6] | N, SdNR, HCSR | TOR 18FG | Osiris 4.18 | 3 (5, 8, 10) | 512 × 512 (8 bits) |
| Gislason et al. [10] | C, CNR | Tin detail | Matlab R2008A | 40 (1–40) | N.R. |
| Vañó et al. [11] | SNR, C, CNR, HCSR | TOR 18FG | Osiris 4.18 | 3 (5, 8, 10) | 512 × 512 (8 bits) |
| Onnasch et al. [7] | SNR | Patient images | N.R. | 1 (1) | 512 × 512 (8 bits) |
| First Author (Reference) | Fluoroscopic System (Detector) | Factor | Categories |
|---|---|---|---|
| Ubeda et al. [1] | Siemens Artis Zee-Zeego (FPD) | PMMA thickness Operation mode | 4, 8, 12, 16, 20 cm FL, FM, FH, CI |
| Bor et al. [12] | Prototype (FPD) | PMMA thickness | 5, 10, 15, 20, 25 cm |
| Anti-scatter grid | With 8:1 and 12:1 covered with carbon fiber, 10:1 and 12:1 covered with aluminum, or without grid | ||
| Tube voltage | 70, 90, 120 kV | ||
| De las Heras et al. [9] | Philips Allura FD 20/10 (FPD) | PMMA thickness | 5.7, 25.7 cm |
| Corredoira et al. [13] | Siemens Artis Zee VC14 (FPD) | Tube voltage Field of view | 4, 8, 12, 16, 20 cm 22, 32, 42, 48 cm |
| Lubis et al. [5] | Philips Allura Xper FD10 (FPD) | Operation mode Vessel diameter Frames per second Contrast mode | FL, FM, FH, CI 0.1, 0.2, 0.4, 0.6, 0.8 cm 15, 30 fps Low, high |
| Ubeda et al. [2] | Siemens Axiom Artis BC (II) | PMMA thickness Operation mode | 4, 8, 12, 16, 20 cm FL, FM, FH, CI |
| Ubeda et al. [4] | Siemens Axiom Artis BC (II) | PMMA thickness Operation mode Anti-scatter grid | 4, 8, 12, 16 cm FL, FM, FH, CI With or without |
| Kordolaimi et al. [19] | Innova 2100 IQ (FPD) Advantx e E/LC þ DLX (II) | PMMA thickness Field of view | 5, 10, 15, 20 cm 12, 15, 17, 20 cm |
| Ubeda et al. [8] | Siemens Axiom Artis dBC (FPD) Philips Allura Xper FD20 (FPD) Toshiba Rebuilt (II) Siemens Axiom Artis BC (II) General Electric Advantx (II) | PMMA thickness Operation mode | 4, 8, 12, 16 cm FL, FM, FH, CI |
| Vañó et al. [20] | Siemens Axiom Artis dBC (FPD) Siemens Axiom Artis FC (II) | PMMA thickness Matrix size | 16, 20, 24, 28 cm 512 × 512, 1024 × 1024 |
| Vañó et al. [6] | Siemens Axiom Artis dBC (FPD) Siemens Axiom Artis BC (II) | PMMA thickness Fluoroscopic system | 8, 12, 16 cm FPD, II |
| Gislason et al. [10] | Allura FD10 (FPD) | PMMA thickness Anti-scatter grid Tube voltage | 8.5, 12, 16 cm With or without 50, 55, 60, 65, 70 kV |
| Vañó et al. [11] | Siemens Axiom Artis BC (II) | PMMA thickness Operation mode Field of view | 4, 8, 12, 16, 20 cm FL, FM, FH, CI 16, 22 |
| Onnasch et al. [7] | Philips Integris BH 5000 (II) | PMMA thickness Anti-scatter grid Tube voltage Filter thickness AEC program | 8, 11, 15.5, 18.5 cm With or without 50–90 kV 0.2, 0.4 mmCu Program (P) from 1 to 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocetti, D.; Villalobos, K.; Wunderle, K. Physical Image Quality Metrics for the Characterization of X-ray Systems Used in Fluoroscopy-Guided Pediatric Cardiac Interventional Procedures: A Systematic Review. Children 2023, 10, 1784. https://doi.org/10.3390/children10111784
Nocetti D, Villalobos K, Wunderle K. Physical Image Quality Metrics for the Characterization of X-ray Systems Used in Fluoroscopy-Guided Pediatric Cardiac Interventional Procedures: A Systematic Review. Children. 2023; 10(11):1784. https://doi.org/10.3390/children10111784
Chicago/Turabian StyleNocetti, Diego, Kathia Villalobos, and Kevin Wunderle. 2023. "Physical Image Quality Metrics for the Characterization of X-ray Systems Used in Fluoroscopy-Guided Pediatric Cardiac Interventional Procedures: A Systematic Review" Children 10, no. 11: 1784. https://doi.org/10.3390/children10111784