Combined Therapy of Vitamin D3-Tolerogenic Dendritic Cells and Interferon-β in a Preclinical Model of Multiple Sclerosis
Abstract
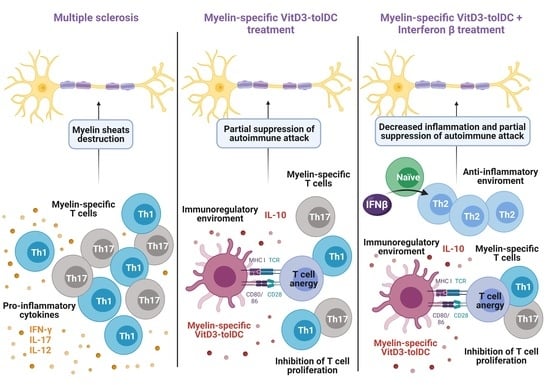
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Monocyte Isolation
2.3. The Generation of Vitamin D3-Tolerogenic Dendritic Cells
2.4. Allogeneic Proliferative Response
2.5. Phenotype
2.5.1. The Phenotype of Monocyte-Derived Dendritic Cells
2.5.2. The Phenotype of Peripheral Blood Mononuclear Cells
2.5.3. The Phenotype of Splenocytes from C57BL/6J Mice
2.6. Animals
2.7. Bone Marrow-Derived Dendritic Cells Generation
2.8. Induction of EAE and Clinical Follow-Up
2.9. In Vivo Treatment of EAE Mice
2.10. Antigen-Specific T Cell Reactivity and Cytokine Secretion
2.11. Statistical Analysis
3. Results
3.1. The Combination of VitD3-tolDC and IFN-Beta Treatment Enhances the Suppressive Ability of VitD3-tolDC and IFN-Beta Monotherapies
3.2. Treatment with VitD3-tolDC Reduces the Activation of T Lymphocytes from RRMS Patients
3.3. VitD3-tolDC+IFN-Beta Culture Induces the Differentiation of Th2 Lymphocytes
3.4. Antigen-Specific VitD3-tolDC Administration in Combination with IFN-Beta Treatment Improves EAE Clinical Signs
3.5. VitD3-tolDC-MOG Treatment Reduces Antigen-Specific T Cell Reactivity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Theofilopoulos, A.N.; Kono, D.H.; Baccala, R. The multiple pathways to autoimmunity. Nat. Immunol. 2017, 18, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, M.J.; Presas-Rodríguez, S.; Teniente-Serra, A.; González-Larreategui, I.; Quirant-Sánchez, B.; Fondelli, F.; Djedovic, N.; Iwaszkiewicz-Grześ, D.; Chwojnicki, K.; Miljković, Đ.; et al. Paving the way towards an effective treatment for multiple sclerosis: Advances in cell therapy. Cell. Mol. Immunol. 2021, 18, 1353–1374. [Google Scholar] [CrossRef]
- Goverman, J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009, 9, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Machado-Santos, J.; Saji, E.; Tröscher, A.; Paunovic, M.; Liblau, R.; Gabriely, G.; Bien, C.G.; Bauer, J.; Lassmann, H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 2018, 141, 2066–2082. [Google Scholar] [CrossRef]
- Xie, Z.-X.; Zhang, H.; Wu, X.-J.; Zhu, J.; Ma, D.-H.; Jin, T. Role of the Immunogenic and Tolerogenic Subsets of Dendritic Cells in Multiple Sclerosis. Mediat. Inflamm. 2015, 2015, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Carlström, K.E.; Ewing, E.; Granqvist, M.; Gyllenberg, A.; Aeinehband, S.; Enoksson, S.L.; Checa, A.; Badam, T.V.S.; Huang, J.; Gomez-Cabrero, D.; et al. Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes. Nat. Commun. 2019, 10, 3081. [Google Scholar] [CrossRef] [Green Version]
- Nuyts, A.; Lee, W.; Bashir-Dar, R.; Berneman, Z.; Cools, N. Dendritic cells in multiple sclerosis: Key players in the immunopathogenesis, key players for new cellular immunotherapies? Mult. Scler. J. 2013, 19, 995–1002. [Google Scholar] [CrossRef] [Green Version]
- Mbongue, J.; Nicholas, D.; Firek, A.; Langridge, W. The Role of Dendritic Cells in Tissue-Specific Autoimmunity. J. Immunol. Res. 2014, 2014, 857143. [Google Scholar] [CrossRef] [Green Version]
- Morante-Palacios, O.; Fondelli, F.; Ballestar, E.; Martínez-Cáceres, E.M. Tolerogenic Dendritic Cells in Autoimmunity and Inflammatory Diseases. Trends Immunol. 2021, 42, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Axtell, R.C.; Raman, C.; Steinman, L. Type I interferons: Beneficial in Th1 and detrimental in Th17 autoimmunity. Clin. Rev. Allergy Immunol. 2013, 44, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirotani, M.; Niino, M.; Fukazawa, T.; Yaguchi, H.; Nakamura, M.; Kikuchi, S.; Sasaki, H. Decreased interferon-α production in response to CpG DNA dysregulates cytokine responses in patients with multiple sclerosis. Clin. Immunol. 2012, 143, 145–151. [Google Scholar] [CrossRef]
- Kim, M.J.; Lim, J.Y.; Park, S.A.; Park, S.I.; Kim, W.S.; Ryu, C.H.; Jeun, S.S. Effective combination of methylprednisolone and interferon β-secreting mesenchymal stem cells in a model of multiple sclerosis. J. Neuroimmunol. 2018, 314, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, L.F.; Acuña, E.; Arellano, G.; Morales, P.; Sotomayor, P.; Oyarzun-Ampuero, F.; Naves, R. Intranasal delivery of interferon-β-loaded nanoparticles induces control of neuroinflammation in a preclinical model of multiple sclerosis: A promising simple, effective, non-invasive, and low-cost therapy. J. Control. Release 2021, 331, 443–459. [Google Scholar] [CrossRef]
- Ten Brinke, A.; Hilkens, C.M.; Cools, N.; Geissler, E.K.; Hutchinson, J.A.; Lombardi, G.; Lord, P.; Sawitzki, B.; Trzonkowski, P.; Van Ham, S.M.; et al. Clinical Use of Tolerogenic Dendritic Cells-Harmonization Approach in European Collaborative Effort. Mediat. Inflamm. 2015, 2015, 471719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, B.E.; Garciafigueroa, Y.; Engman, C.; Trucco, M.; Giannoukakis, N. Tolerogenic Dendritic Cells and T-Regulatory Cells at the Clinical Trials Crossroad for the Treatment of Autoimmune Disease; Emphasis on Type 1 Diabetes Therapy. Front. Immunol. 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, G.M.; Anderson, A.E.; Diboll, J.; Reece, R.; Eltherington, O.; Harry, R.A.; Fouweather, T.; MacDonald, C.; Chadwick, T.; McColl, E.; et al. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann. Rheum. Dis. 2017, 76, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Fousteri, G.; Jofra, T.; Di Fonte, R. Combination of an Antigen-Specific Therapy and an Immunomodulatory Treatment to Simultaneous Block Recurrent Autoimmunity and Alloreactivity in Non-Obese Diabetic Mice. PLoS ONE 2015, 10, e0127631. [Google Scholar] [CrossRef] [Green Version]
- Northrup, L.; Christopher, M.A.; Sullivan, B.P.; Berkland, C. Combining antigen and immunomodulators: Emerging trends in antigen-specific immunotherapy for autoimmunity. Adv. Drug Deliv. Rev. 2016, 98, 86–98. [Google Scholar] [CrossRef]
- Flórez-Grau, G.; Zubizarreta, I.; Cabezón, R.; Villoslada, P.; Benitez-Ribas, D. Tolerogenic Dendritic Cells as a Promising Antigen-Specific Therapy in the Treatment of Multiple Sclerosis and Neuromyelitis Optica from Preclinical to Clinical Trials. Front. Immunol. 2018, 31, 1169. [Google Scholar] [CrossRef] [Green Version]
- Torkildsen, Ø.; Myhr, K.M.; Bø, L. Disease-modifying treatments for multiple sclerosis-a review of approved medications. Eur. J. Neurol. 2016, 23, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Pardo, G.; Jones, D.E. The sequence of disease-modifying therapies in relapsing multiple sclerosis: Safety and immunologic considerations. J. Neurol. 2017, 264, 2351–2374. [Google Scholar] [CrossRef] [Green Version]
- Willekens, B.L.; Presas-Rodríguez, S.; Mansilla, M.J.; Derdelinckx, J.; Lee, W.-P.; Nijs, G.; De Laere, M.; Wens, I.; Cras, P.; Parizel, P.; et al. Tolerogenic dendritic cell-based treatment for multiple sclerosis (MS): A harmonised study protocol for two phase I clinical trials comparing intradermal and intranodal cell administration. BMJ Open 2019, 9, e030309. [Google Scholar] [CrossRef] [Green Version]
- Derdelinckx, J.; Mansilla, M.J.; De Laere, M.; Lee, W.P.; Navarro-Barriuso, J.; Wens, I.; Nkansah, I.; Daans, J.; De Reu, H.; Jolanta Keliris, A.; et al. Clinical and immunological control of experimental autoimmune encephalomyelitis by tolerogenic dendritic cells loaded with MOG-encoding mRNA. J. Neuroinflamm. 2019, 16, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansilla, M.J.; Sellès-Moreno, C.; Fàbregas-Puig, S. Beneficial Effect of Tolerogenic Dendritic Cells Pulsed with MOG Autoantigen in Experimental Autoimmune Encephalomyelitis. CNS Neurosci. Ther. 2015, 21, 222–230. [Google Scholar] [CrossRef]
- Mansilla, M.J.; Contreras-Cardone, R.; Navarro-Barriuso, J. Cryopreserved vitamin D3-tolerogenic dendritic cells pulsed with autoantigens as a potential therapy for multiple sclerosis patients. J. Neuroinflamm. 2016, 13, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten Brinke, A.; Martinez-Llordella, M.; Cools, N. Ways Forward for Tolerance-Inducing Cellular Therapies- an AFACTT Perspective. Front. Immunol. 2019, 10, 181. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Barriuso, J.; Mansilla, M.J.; Quirant-Sánchez, B.; Ardiaca-Martínez, A.; Teniente-Serra, A.; Presas-Rodríguez, S.; Ten Brinke, A.; Ramo-Tello, C.; Martínez-Cáceres, E.M. MAP7 and MUCL1 Are Biomarkers of Vitamin D3-Induced Tolerogenic Dendritic Cells in Multiple Sclerosis Patients. Front. Immunol. 2019, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Quirant-Sánchez, B.; Hervás-García, J.V.; Teniente-Serra, A.; Brieva, L.; Moral-Torres, E.; Cano, A.; Munteis, E.; Mansilla, M.J.; Presas-Rodriguez, S.; Navarro-Barriuso, J.; et al. Predicting therapeutic response to fingolimod treatment in multiple sclerosis patients. CNS Neurosci. Ther. 2018, 24, 1175–1184. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Matsoukas, J. Advances in Multiple Sclerosis Research-Series I. Brain Sci. 2020, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef]
- Polman, C.H.; O’Connor, P.W.; Havrdova, E.; Hutchinson, M.; Kappos, L.; Miller, D.H.; Phillips, J.T.; Lublin, F.D.; Giovannoni, G.; Wajgt, A.; et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 2006, 354, 899–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, S.L.; Waubant, E.; Arnold, D.L. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008, 358, 676–688. [Google Scholar] [CrossRef] [Green Version]
- Giovannoni, G.; Comi, G.; Cook, S.; Rammohan, K.; Rieckmann, P.; Soelberg Sørensen, P.; Vermersch, P.; Chang, P.; Hamlett, A.; Musch, B.; et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Kappos, L.; Radue, E.W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, P.; Wolinsky, J.S.; Confavreux, C.; Comi, G.; Kappos, L.; Olsson, T.P.; Benzerdjeb, H.; Truffinet, P.; Wang, L.; Miller, A.; et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N. Engl. J. Med. 2011, 365, 1293–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.A.; Coles, A.J.; Arnold, D.L.; Confavreux, C.; Fox, E.J.; Hartung, H.P.; Havrdova, E.; Selmaj, K.W.; Weiner, H.L.; Fisher, E.; et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet 2012, 380, 1819–1828. [Google Scholar] [CrossRef]
- Calabresi, P.A.; Radue, E.W.; Goodin, D.; Jeffery, D.; Rammohan, K.W.; Reder, A.T.; Vollmer, T.; Agius, M.A.; Kappos, L.; Stites, T.; et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014, 13, 545–556. [Google Scholar] [CrossRef]
- Wang, Q.; Chuikov, S.; Taitano, S.; Wu, Q.; Rastogi, A.; Tuck, S.J.; Corey, J.M.; Lundy, S.K.; Mao-Draayer, Y. Dimethyl Fumarate Protects Neural Stem/Progenitor Cells and Neurons from Oxidative Damage through Nrf2-ERK1/2 MAPK Pathway. Int. J. Mol. Sci. 2015, 16, 13885–13907. [Google Scholar] [CrossRef] [Green Version]
- Hauser, S.L.; Bar-Or, A.; Comi, G. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, M.J.; Navarro-Barriuso, J.; Presas-Rodríguez, S.; Teniente-Serra, A.; Quirant-Sánchez, B.; Ramo-Tello, C.; Martínez-Cáceres, E.M. Optimal response to dimethyl fumarate is mediated by a reduction of Th1-like Th17 cells after 3 months of treatment. CNS Neurosci. Ther. 2019, 25, 995–1005. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef]
- Kolb-Mäurer, A.; Goebeler, M.; Mäurer, M. Cutaneous Adverse Events Associated with Interferon-β Treatment of Multiple Sclerosis. Int. J. Mol. Sci. 2015, 16, 14951–14960. [Google Scholar] [CrossRef] [Green Version]
- Berger, J.R.; Cree, B.A.; Greenberg, B.; Hemmer, B.; Ward, B.J.; Dong, V.M.; Merschhemke, M. Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology 2018, 90, e1815–e1821. [Google Scholar] [CrossRef] [Green Version]
- Mills, E.A.; Mao-Draayer, Y. Aging and lymphocyte changes by immunomodulatory therapies impact PML risk in multiple sclerosis patients. Mult. Scler. J. 2018, 24, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Ryerson, L.Z.; Foley, J.; Chang, I.; Kister, I.; Cutter, G.; Metzger, R.R.; Goldberg, J.D.; Li, X.; Riddle, E.; Smirnakis, K.; et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology 2019, 93, e1452–e1462. [Google Scholar] [CrossRef] [Green Version]
- Focosi, D.; Tuccori, M.; Maggi, F. Progressive multifocal leukoencephalopathy and anti-CD20 monoclonal antibodies: What do we know after 20 years of rituximab. Rev. Med. Virol. 2019, 29, e2077. [Google Scholar] [CrossRef]
- Medaer, R.; Stinissen, P.; Truyen, L.; Raus, J.; Zhang, J. Depletion of myelin-basic-protein autoreactive T cells by T-cell vaccination: Pilot trial in multiple sclerosis. Lancet 1995, 346, 807–808. [Google Scholar] [CrossRef]
- Hu, J.; Wan, Y. Tolerogenic dendritic cells and their potential applications. Immunology 2011, 132, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Lutterotti, A.; Yousef, S.; Sputtek, A.; Stürner, K.H.; Stellmann, J.P.; Breiden, P.; Reinhardt, S.; Schulze, C.; Bester, M.; Heesen, C.; et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: A phase 1 trial in multiple sclerosis. Sci. Transl. Med. 2013, 5, 188ra75. [Google Scholar] [CrossRef] [Green Version]
- Calabresi, P.A.; Wilterdink, J.L.; Rogg, J.M.; Mills, P.; Webb, A.; Whartenby, K.A. An open-label trial of combination therapy with interferon beta-1a and oral methotrexate in MS. Neurology 2002, 58, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Pulicken, M.; Bash, C.N.; Costello, K.; Said, A.; Cuffari, C.; Wilterdink, J.L.; Rogg, J.M.; Mills, P.; Calabresi, P.A. Optimization of the safety and efficacy of interferon beta 1b and azathioprine combination therapy in multiple sclerosis. Mult. Scler. J. 2005, 11, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, D.R.; Chepuri, N.; Durden, D.; Burdette, J. A pilot trial of combination therapy with mitoxantrone and interferon beta-1b using monthly gadolinium-enhanced magnetic resonance imaging. Mult. Scler. J. 2005, 11, 296–301. [Google Scholar] [CrossRef]
- Rudick, R.A.; Stuart, W.H.; Calabresi, P.A.; Confavreux, C.; Galetta, S.L.; Radue, E.W.; Lublin, F.D.; Weinstock-Guttman, B.; Wynn, D.R.; Lynn, F.; et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N. Engl. J. Med. 2006, 354, 911–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lublin, F.D.; Cofield, S.S.; Cutter, G.R.; Conwit, R.; Narayana, P.A.; Nelson, F.; Salter, A.R.; Gustafson, T.; Wolinsky, J.S.; CombiRx Investigators. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann. Neurol. 2013, 73, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Macedo, C.; Turquist, H.; Metes, D.; Thomson, A.W. Immunoregulatory properties of rapamycin-conditioned monocyte-derived dendritic cells and their role in transplantation. Transplant. Res. 2012, 1, 16. [Google Scholar] [CrossRef] [Green Version]
- Suwandi, J.S.; Toes, R.E.; Nikolic, T.; Roep, B.O. Inducing tissue specific tolerance in autoimmune disease with tolerogenic dendritic cells. Clin. Exp. Rheumatol. 2015, 33, S97–S103. [Google Scholar]
- Nguyen-Pham, T.N.; Jung, S.H.; Vo, M.C.; Thanh-Tran, H.T.; Lee, Y.K.; Lee, H.J.; Choi, N.R.; Hoang, M.D.; Kim, H.J.; Lee, J.J. Lenalidomide Synergistically Enhances the Effect of Dendritic Cell Vaccination in a Model of Murine Multiple Myeloma. J. Immunother. 2015, 38, 330–339. [Google Scholar] [CrossRef]
- Zhou, Y.; Shan, J.; Guo, Y.; Li, S.; Long, D.; Li, Y.; Feng, L. Effects of Adoptive Transfer of Tolerogenic Dendritic Cells on Allograft Survival in Organ Transplantation Models: An Overview of Systematic Reviews. J. Immunol. Res. 2016, 2016, 5730674. [Google Scholar] [CrossRef] [Green Version]
- Dáňová, K.; Grohová, A.; Strnadová, P.; Funda, D.P.; Šumník, Z.; Lebl, J.; Cinek, O.; Průhová, Š.; Koloušková, S.; Obermannová, B.; et al. Tolerogenic Dendritic Cells from Poorly Compensated Type 1 Diabetes Patients Have Decreased Ability To Induce Stable Antigen-Specific T Cell Hyporesponsiveness and Generation of Suppressive Regulatory T Cells. J. Immunol. 2017, 198, 729–740. [Google Scholar] [CrossRef] [Green Version]
- Grau-López, L.; Granada, M.L.; Raïch-Regué, D.; Naranjo-Gómez, M.; Borràs-Serres, F.E.; Martínez-Cáceres, E.; Ramo-Tello, C. Regulatory role of vitamin D in T-cell reactivity against myelin peptides in relapsing-remitting multiple sclerosis patients. BMC Neurol. 2012, 12, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramgolam, V.S.; Sha, Y.; Jin, J.; Zhang, X.; Markovic-Plese, S. IFN-beta inhibits human Th17 cell differentiation. J. Immunol. 2009, 183, 5418–5427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinschek, B.; Luessi, F.; Gross, C.C.; Wiendl, H.; Jonuleit, H. Interferon-Beta Therapy of Multiple Sclerosis Patients Improves the Responsiveness of T Cells for Immune Suppression by Regulatory T Cells. Int. J. Mol. Sci. 2015, 16, 16330–16346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Vécsei, L. Monitoring the Redox Status in Multiple Sclerosis. Biomedicines 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]






| Condition | RRMS Patients (n = 6) | HD (n = 6) | p-Value | ||
|---|---|---|---|---|---|
| Mean (%) | SD | Mean (%) | SD | ||
| 100%VitD3-tolDC | 54.73 | 9.90 | 56.14 | 18.43 | 0.87 |
| 100%VitD3-tolDC + IFN-beta | 64.87 | 8.28 | 61.14 | 16.17 | 0.62 |
| 75%VitD3-tolDC: 25%mDC | 42.63 | 23.95 | 49.29 | 8.81 | 0.51 |
| 75%VitD3-tolDC: 25%mDC + IFN-beta | 57.05 | 20.09 | 65.29 | 12.97 | 0.39 |
| 50%VitD3-tolDC: mDC | 21.00 | 16.00 | 36.29 | 10.44 | 0.14 |
| 50%VitD3-tolDC: mDC + IFN-beta | 25.00 | 19.00 | 45.71 | 19.54 | 0.16 |
| 25%VitD3-tolDC: 75%mDC | 12.00 | 17.00 | 19.23 | 13.99 | 0.25 |
| 25%VitD3-tolDC: 75%mDC + IFN-beta | 28.00 | 6.78 | 40.86 | 13.83 | 0.10 |
| Condition | RRMS n = 6 | HD n = 6 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Mean (%) | SD | p-Value | Mean (%) | SD | |||
| CD4 Activated lymphocytes ** | mDC | 14.03 | 3.73 | - | 11.50 | 8.11 | - |
| mDC + IFN-beta | 12.68 | 4.92 | 0.604 | 9.28 | 5.95 | 0.514 | |
| 100%VitD3-tolDC | 4.95 | 1.71 | 0.003 | 5.16 | 2.60 | 0.050 | |
| 100%VitD3-tolDC + IFN-beta | 4.27 | 1.68 | 0.003 | 5.25 | 2.42 | 0.060 | |
| 50% VitD3-tolDC | 8.13 | 3.58 | 0.020 | 8.01 | 4.85 | 0.310 | |
| 50% VitD3-tolDC + IFN-beta | 10.10 | 4.77 | 0.150 | 7.21 | 4.63 | 0.210 | |
| CD8 Activated lymphocytes * | mDC | 11.33 | 5.11 | - | 14.33 | 8.82 | - |
| mDC + IFN-beta | 10.60 | 6.07 | 0.825 | 13.83 | 8.37 | 0.028 | |
| 100%VitD3-tolDC | 3.72 | 2.12 | 0.007 | 5.07 | 4.50 | 0.040 | |
| 100%VitD3-tolDC + IFN-beta | 3.33 | 1.56 | 0.004 | 4.75 | 2.55 | 0.020 | |
| 50% VitD3-tolDC | 7.28 | 1.21 | 0.087 | 10.70 | 7.27 | 0.450 | |
| 50% VitD3-tolDC + IFN-beta | 8.70 | 4.11 | 0.340 | 7.62 | 2.26 | 0.100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quirant-Sánchez, B.; Mansilla, M.J.; Navarro-Barriuso, J.; Presas-Rodríguez, S.; Teniente-Serra, A.; Fondelli, F.; Ramo-Tello, C.; Martínez-Cáceres, E. Combined Therapy of Vitamin D3-Tolerogenic Dendritic Cells and Interferon-β in a Preclinical Model of Multiple Sclerosis. Biomedicines 2021, 9, 1758. https://doi.org/10.3390/biomedicines9121758
Quirant-Sánchez B, Mansilla MJ, Navarro-Barriuso J, Presas-Rodríguez S, Teniente-Serra A, Fondelli F, Ramo-Tello C, Martínez-Cáceres E. Combined Therapy of Vitamin D3-Tolerogenic Dendritic Cells and Interferon-β in a Preclinical Model of Multiple Sclerosis. Biomedicines. 2021; 9(12):1758. https://doi.org/10.3390/biomedicines9121758
Chicago/Turabian StyleQuirant-Sánchez, Bibiana, María José Mansilla, Juan Navarro-Barriuso, Silvia Presas-Rodríguez, Aina Teniente-Serra, Federico Fondelli, Cristina Ramo-Tello, and Eva Martínez-Cáceres. 2021. "Combined Therapy of Vitamin D3-Tolerogenic Dendritic Cells and Interferon-β in a Preclinical Model of Multiple Sclerosis" Biomedicines 9, no. 12: 1758. https://doi.org/10.3390/biomedicines9121758