The Biology of Vasopressin
Abstract
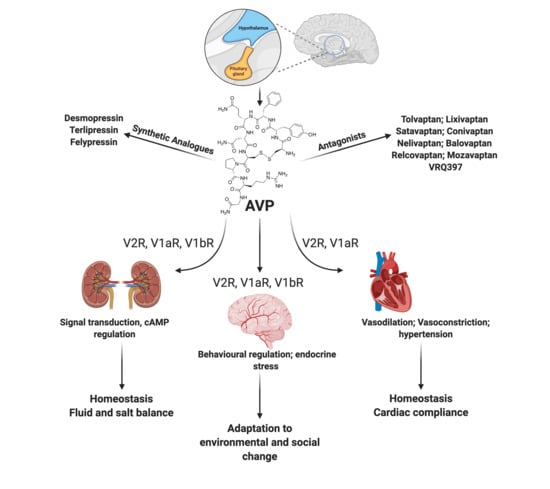
:1. Introduction
1.1. Early Discoveries
1.2. AVP Function
1.3. AVP Gene Expression
1.4. AVP Synthesis
1.5. AVP Receptors
1.5.1. Kidney
1.5.2. Heart
1.5.3. Brain
1.5.4. Other Tissues
1.5.5. Regulation of AVP Receptor Expression
1.5.6. Receptor Desensitization
2. AVP-Related Renal Pathology
2.1. Polycystic Kidney Disease and AVP
2.2. Biological Sex and PKD
3. AVP and Heart Failure
3.1. AVP-V1aR Signaling and Cardiac Contractility
3.2. AVP-V1aR Signaling and Cardiac Remodeling
3.3. AVP-V2R Signaling for Fluid Volume Retention and Cardiac Function
4. AVP and Brain Function
4.1. AVP and Animal Behavior
4.1.1. V1bR and Behavior
4.1.2. V1aR and Behavior
4.1.3. Recognition
4.1.4. Aggression
4.1.5. Parental Care
4.1.6. Sexual Behaviors
4.1.7. Differential Sex Response to AVP
4.1.8. Changes in AVP-Signaling
4.1.9. Circadian Response
4.2. AVP and Human Behaviour
5. Pharmacological Modulation of the AVP Pathways
5.1. Vasopressin Receptor Antagonists
5.1.1. V2R Antagonism in Heart Disease Therapy
5.1.2. V2R Antagonism in PKD Therapy
5.1.3. Clinical Trials
5.2. Synthetic AVP Analogs
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gamberi, C.; Hall, K. Undergraduates can publish too! A case study of a scientific team writing assignment leading to publication. Int. J. Sci. Educ. 2019, 41, 48–63. [Google Scholar]
- Selber-Hnatiw, S.; Rukundo, B.; Ahmadi, M.; Akoubi, H.; Al-Bizri, H.; Aliu, A.F.; Ambeaghen, T.U.; Avetisyan, L.; Bahar, I.; Baird, A.; et al. Human gut microbiota: Toward an ecology of disease. Front. Microbiol. 2017, 8, 1265. [Google Scholar] [CrossRef]
- Selber-Hnatiw, S.; Sultana, T.; Tse, W.; Abdollahi, N.; Abdullah, S.; Al Rahbani, J.; Alazar, D.; Alrumhein, N.J.; Aprikian, S.; Arshad, R.; et al. Metabolic networks of the human gut microbiota. Microbiology 2020, 166, 96–119. [Google Scholar] [CrossRef]
- Hoyle, C.H. Neuropeptide families and their receptors: Evolutionary perspectives. Brain Res. 1999, 848, 1–25. [Google Scholar] [CrossRef]
- Boone, M.; Deen, P.M. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflugers Arch. 2008, 456, 1005–1024. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T.M.; Bichet, D.G. Molecular biology of hereditary diabetes insipidus. J. Am. Soc. Nephrol. 2005, 16, 2836–2846. [Google Scholar] [CrossRef]
- Farini, F. Diabete insipido ed opoterapia. Gazz. Osped. Clin. 1913, 34, 1135–1139. [Google Scholar]
- Qureshi, S.; Galiveeti, S.; Bichet, D.G.; Roth, J. Diabetes insipidus: Celebrating a century of vasopressin therapy. Endocrinology 2014, 155, 4605–4621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vongraven, D. Die nierenwirkung von hypophysenextrakten meschen. Berl. Klin. Wochenscgr. 1913, 50, 2083–2086. [Google Scholar]
- du Vigneaud, V.; Gish, D.T.; Katsoyannis, P.G. A synthetic preparation possessing biological properties associated with arginine-vasopressin. J. Am. Chem. Soc. 1954, 76, 4751–4752. [Google Scholar] [CrossRef]
- du Vigneaud, V.; Ressler, C.; Swan, J.M.; Roberts, C.W.; Katsoyannis, P.G. The synthesis of oxytocin. J. Am. Chem. Soc. 1954, 76, 3115–3121. [Google Scholar] [CrossRef]
- Birnbaumer, M.; Seibold, A.; Gilbert, S.; Ishido, M.; Barberis, C.; Antaramian, A.; Brabet, P.; Rosenthal, W. Molecular cloning of the receptor for human antidiuretic hormone. Nature 1992, 357, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Tanizawa, O.; Mori, K.; Brownstein, M.J.; Okayama, H. Structure and expression of a human oxytocin receptor. Nature 1992, 356, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Lolait, S.J.; O’Carroll, A.M.; McBride, O.W.; Konig, M.; Morel, A.; Brownstein, M.J. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature 1992, 357, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.; O’Carroll, A.M.; Brownstein, M.J.; Lolait, S.J. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature 1992, 356, 523–526. [Google Scholar] [CrossRef]
- De Keyzer, Y.; Auzan, C.; Lenne, F.; Beldjord, C.; Thibonnier, M.; Bertagna, X.; Clauser, E. Cloning and characterization of the human V3 pituitary vasopressin receptor. FEBS Lett. 1994, 356, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, T.; Saito, M.; Mochizuki, S.; Watanabe, Y.; Hashimoto, S.; Kawashima, H. Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J. Biol. Chem. 1994, 269, 27088–27092. [Google Scholar] [CrossRef]
- Thibonnier, M.; Auzan, C.; Madhun, Z.; Wilkins, P.; Berti-Mattera, L.; Clauser, E. Molecular cloning, sequencing, and functional expression of a cDNA encoding the human V1a vasopressin receptor. J. Biol. Chem. 1994, 269, 3304–3310. [Google Scholar] [CrossRef]
- Rozen, F.; Russo, C.; Banville, D.; Zingg, H.H. Structure, characterization, and expression of the rat oxytocin receptor gene. Proc. Natl. Acad. Sci. USA 1995, 92, 200–204. [Google Scholar] [CrossRef] [Green Version]
- Mckinley, M.; Mathai, M.; McAllen, R.; McClear, R.; Miselis, R.; Pennington, G.L.; Vivas, L.; Wade, J.D.; Oldfield, B.J. Vasopressin secretion: Osmotic and hormonal regulation by the lamina terminalis. J. Neuroendocrinol. 2004, 16, 340–347. [Google Scholar] [CrossRef]
- Bourque, C.W.; Oliet, S.R.; Richard, D. Osmoreceptors, osmoreception, and osmoregulation. Front. Neuroendocrinol. 1994, 15, 231–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W. Hypernatemia: Successful treatment. Electrolyte Blood Press. 2006, 4, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Bichet, D.G. Central vasopressin: Dendritic and axonal secretion and renal actions. Clin. Kidney J. 2014, 7, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gastel, M.D.A.; Torres, V.E. Polycystic kidney disease and the vasopressin pathway. Ann. Nutr. Metab. 2017, 70, 43–50. [Google Scholar] [CrossRef]
- Koshimizu, T.A.; Nakamura, K.; Egashira, N.; Hiroyama, M.; Nonoguchi, H.; Tanoue, A. Vasopressin V1a and V1b receptors: From molecules to physiological systems. Physiol. Rev. 2012, 92, 1813–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuzzo, B.; Padala, S.A.; Lappin, S.L. Vasopressin (antidiuretic hormone, ADH). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Song, Z.; Albers, H.E. Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Front. Neuroendocrinol. 2018, 51, 14–24. [Google Scholar] [CrossRef]
- Francis, G.S.; Goldsmith, S.R.; Levine, B.; Olivari, M.T.; Cohn, J.N. The neurohumoral axis in congestive heart failure. Ann. Intern. Med. 1984, 101, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.R.; Francis, G.S.; Cowley, A.W., Jr.; Levine, T.B.; Cohn, J.N. Increased plasma arginine vasopressin levels in patients with con-gestive heart failure. J. Am. Coll. Cardiol. 1983, 1, 1385–1390. [Google Scholar] [CrossRef] [Green Version]
- Riegger, G.A.J.; Liebeau, G.; Kochsiek, K. Antidiuretic hormone in congestive heart failure. Am. J. Med. 1982, 72, 49–52. [Google Scholar] [CrossRef]
- Dumais, K.M.; Veenema, A.H. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front. Neuroendocrinol. 2016, 40, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Goodson, J.L.; Thompson, R.R. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr. Opin. Neurobiol. 2010, 20, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, M.; von Dawans, B.; Domes, G. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocrinol. 2009, 30, 548–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zink, C.F.; Kempf, L.; Hakimi, S.; Rainey, C.A.; Stein, J.L.; Meyer-Lindenberg, A. Vasopressin modulates social recognition-related activity in the left temporoparietal junction in humans. Transl. Psychiatry 2011, 1, e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, Y.; Oiso, Y.; Saito, H.; Majzoub, J.A. Positive and negative regulation of the rat vasopressin gene promoter. Endocrinology 1997, 138, 5266–5274. [Google Scholar] [CrossRef]
- Kuwahara, S.; Arima, H.; Banno, R.; Sato, I.; Kondo, N.; Oiso, Y. Regulation of vasopressin gene expression by cAMP and glucocorticoids in parvocellular neurons of the paraventricular nucleus in rat hypothalamic organotypic cultures. J. Neurosci. 2003, 23, 10231–10237. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M. Gene regulation system of vasopressin and corticotropin-releasing hormone. Gene Regul. Syst. Bio. 2008, 2, 71–88. [Google Scholar]
- Ball, S.G. The neurohypophysis: Endocrinology of vasopressin and oxytocin. In Endotext [internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., Kaltsas, G., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2017; p. 2017. [Google Scholar]
- Mohr, E.; Kachele, I.; Mullin, C.; Richter, D. Rat vasopressin mRNA: A model system to characterize cis-acting elements and trans-acting factors in dendritic mRNA sorting. Prog. Brain Res. 2001, 139, 211–224. [Google Scholar]
- Zingg, H.H.; Lefebre, D.L.; Almazan, G. Regulation of poly(A) tail size of vasopressin mRNA. J. Biol. Chem. 1988, 15, 11041–11043. [Google Scholar] [CrossRef]
- Preiss, T.; Brakier-Gingras, L.; Lapointe, J. The end in sight: Poly(A), translation and mRNA stability in eukaryotes. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2000; pp. 2000–2013. [Google Scholar]
- Rotondo, F.; Butz, H.; Syro, L.V.; Yousef, G.M.; Di leva, A.; Restrepo, L.M.; Quintanar-Stephano, A.; Berczi, I.; Kovacs, K. Arginine vasopressin (AVP): A review of its historical perspectives, current research and multifunctional role in the hypothalamo-hypophysial system. Pituitary 2016, 19, 345–355. [Google Scholar] [CrossRef]
- Morales-Medina, J.C.; Witchey, S.K.; Caldwell, H.K. The role of vasopressin in anxiety and depression. In Melatonin, Neuroprotective Agents and Antidepressant Therapy; López-Muñoz, F., Srinivasan, V., de Berardis, D., Álamo, C., Kato, T.A., Eds.; Springer: New Delhi, India, 2016; pp. 667–685. [Google Scholar]
- Waller, S.J.; Ratty, A.; Burbach, J.P.H.; Murphy, D. Transgenic and transcriptional studies on neurosecretory cell gene expression. Cell. Mol. Neurobiol. 1998, 18, 149–171. [Google Scholar] [CrossRef]
- Acher, R.; Chauvet, J.; Rouille, Y. Dynamic processing of neuropeptides. J. Mol. Neurosci. 2002, 18, 223–228. [Google Scholar] [CrossRef]
- Perucca, J.; Bouby, N.; Valeix, P.; Bankir, L. Sex difference in urine concentration across differing ages, sodium intake, and level of kidney disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R700–R705. [Google Scholar] [CrossRef] [PubMed]
- Roussel, R.; Fezeu, L.; Marre, M.; Velho, G.; Fumeron, F.; Jungers, P.; Lantieri, O.; Balkau, B.; Bouby, N.; Bankir, L.; et al. Comparison between copeptin and vasopressin in a population from the community and in people with chronic kidney disease. J. Clin. Endocrinol. Metab. 2014, 99, 4656–4663. [Google Scholar] [CrossRef] [PubMed]
- Barberis, C.; Mouillac, B.; Durroux, T. Structural bases of vasopressin/oxytocin receptor function. J. Endocrinol. 1998, 156, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Baumann, G.; Dingman, J.F. Distribution, blood transport, and degradation of antidiuretic hormone in man. J. Clin. Investig. 1976, 57, 1109–1116. [Google Scholar] [CrossRef]
- Hoffert, J.D.; Pisitkun, T.; Saeed, F.; Song, J.H.; Chou, C.L.; Knepper, M.A. Dynamics of the G protein-coupled vasopressin V2 receptor signaling network revealed by quantitative phosphoproteomics. Mol. Cell. Proteomics 2012, 11, M111.014613. [Google Scholar] [CrossRef] [Green Version]
- Bastian, F.B.; Roux, J.; Niknejad, A.; Comte, A.; Costa, S.S.F.; de Farias, T.M.; Moretti, S.; Parmentier, G.; de Laval, V.R.; Rosikiewicz, M.; et al. The Bgee suite: Integrated curated expression atlas and comparative transcriptomics in animals. Nucl. Acids Res. 2020, gkaa793. [Google Scholar] [CrossRef]
- Young, W.S., III; Kovacs, K.; Lolait, S.J. The diurnal rhythm in vasopressin V1a receptor expression in the suprachiasmatic nucleus is not dependent on vasopressin. Endocrinology 1993, 133, 585–590. [Google Scholar] [CrossRef]
- Greenberg, A.; Verbalis, J.G. Vasopressin receptor antagonists. Kidney Int. 2006, 69, 2124–2130. [Google Scholar] [CrossRef] [Green Version]
- Holmes, C.L.; Patel, B.M.; Russell, J.A.; Walley, K.R. Physiology of vasopressin relevant to management of septic shock. Chest 2001, 120, 989–1002. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Igarashi, N.; Hirasawa, A.; Tsujimoto, G.; Kobayashi, M. Distribution and developmental changes in vasopressin V2 receptor mRNA in rat brain. Differentiation 1995, 59, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Mutig, K.; Paliege, A.; Kahl, T.; Jons, T.; Muller-Esterl, W.; Bachmann, S. Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am. J. Physiol. Renal Physiol. 2007, 293, F1166–F1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmosino, M.; Brooks, H.L.; Cai, Q.; Davis, L.S.; Opalenik, S.; Hao, C.; Breyer, M.D. Axial heterogeneity of vasopressin-receptor subtypes along the human and mouse collecting duct. Am. J. Physiol. Renal Physiol. 2007, 292, F351–F360. [Google Scholar] [CrossRef] [PubMed]
- Hirashawa, A.; Shibata, K.; Kotosai, K.; Tsujimoto, G. Cloning, functional expression and tissue distribution of human cDNA for the vascular-type vasopressin receptor. Biochem. Biophys. Res. Commun. 1994, 203, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Park, F.; Mattson, D.L.; Skelton, M.M.; Cowley, A.W., Jr. Localization of the vasopressin V1a and V2 receptors within the renal cortical and medullary circulation. Am. J. Physiol. 1997, 273, R243–R251. [Google Scholar] [CrossRef]
- Saito, M.; Tahara, A.; Sugimoto, T.; Abe, K.; Furuichi, K. Evidence that atypical vasopressin V2 receptor in inner medulla of kidney is V1b receptor. Eur. J. Pharmacol. 2000, 401, 289–296. [Google Scholar] [CrossRef]
- Gozdz, A.; Szczepanska-Sadowska, E.; Szczepanska, K.; Maslinski, W.; Luszczyk, B. Vasopressin V1a, V1b and V2 receptors mRNA in the kidney and heart of the renin transgenic TGR(mRen2)27 and Sprague Dawley rats. J. Physiol. Pharmacol. 2002, 53, 349–357. [Google Scholar]
- Cottet-Maire, F.; Avdonin, P.V.; Roulet, E.; Buetler, T.M.; Mermod, N.; Ruegg, U.T. Upregulation of vasopressin V1a receptor mRNA and protein in vascular smooth muscle cells following cyclosporin A treatment. Br. J. Pharmacol. 2001, 132, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Verbalis, J.G. Vasopressin receptors. In Molecular Mechanisms of Hormone Actions on Behavior, 1st ed.; Etgen, A.M., Pfaff, D.W., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 803–810. [Google Scholar]
- Jasnic, N.; Djordjevic, J.; Vujovic, P.; Lakic, I.; Djurasevic, S.; Cvijic, G. The effect of vasopressin 1b receptor (V1bR) blockade on HPA axis activity in rats exposed to acute heat stress. J. Exp. Biol. 2013, 216, 2303–2307. [Google Scholar] [CrossRef] [Green Version]
- El-Werfali, W.; Toomasian, C.; Maliszewska-Scislo, M.; Li, C.; Rossi, N.F. Haemodynamic and renal sympathetic responses to V1b vasopressin receptor activation within the paraventricular nucleus. Exp. Physiol. 2015, 100, 553–565. [Google Scholar] [CrossRef]
- Ahn, D.K.; Kim, K.H.; Ju, J.S.; Kwon, S.; Park, J.S. Microinjection of arginine vasopressin into the central nucleus of amygdala suppressed nociceptive jaw opening reflex in freely moving rats. Brain Res. Bull. 2001, 55, 117–121. [Google Scholar] [CrossRef]
- Carter, C.S. The oxytocin-vasopressin pathway in the context of love and fear. Front. Endocrinol. (Lausanne) 2017, 8, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, Z.V.; Young, L.J. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev. 2017, 76, 87–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, L.R.; Suberg, S.N.; Thurston, C.L.; Culhane, E.S. Role of spinal cord neuropeptides in pain sensitivity and analgesia: Thyrotropin releasing hormone and vasopressin. Brain Res. 1986, 362, 308–317. [Google Scholar] [CrossRef]
- Pasquali, R.; Gagliardi, L.; Vicennati, V.; Gambineri, A.; Colitta, D.; Ceroni, L.; Casimirri, F. ACTH and cortisol response to combined corticotropin releasing hormone-arginine vasopressin stimulation in obese males and its relationship to body weight, fat distribution and parameters of the metabolic syndrome. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 419–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.D.; Burton, K.J.; Zhang, C.; Hu, S.B.; Zhou, Q.Y. Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R824–R830. [Google Scholar] [CrossRef] [Green Version]
- Folny, V.; Raufaste, D.; Lukovic, L.; Pouzet, B.; Rochard, P.; Pascal, M.; Serradeil-Le Gal, C. Pancreatic vasopressin V1b receptors: Characterization in In-R1-G9 cells and localization in human pancreas. Am. J. Physiol. Endocrinol. Metab. 2003, 285, 566–576. [Google Scholar] [CrossRef] [Green Version]
- Bichet, D.G.; Arthus, M.F.; Lonergan, M. Platelet vasopressin receptors in patients with congenital nephrogenic diabetes insipidus. Kidney Int. 1991, 39, 693–699. [Google Scholar] [CrossRef] [Green Version]
- Baker, C.; Richards, L.J.; Dayan, C.M.; Jessop, D.S. Corticotropin-releasing hormone immunoreactivity in human T and B cells and macrophages: Colocalization with arginine vasopressin. J. Neuroendocrinol. 2003, 15, 1070–1074. [Google Scholar] [CrossRef]
- Wiedermann, F.J.; Watzinger, K.; Stichlberger, M.; Joannidis, M.; Kaehler, C.; Lederer, W. Effects of arginine vasopressin on migration and respiratory burst activity in human leukocytes. Open Med. (Wars) 2018, 13, 122–129. [Google Scholar] [CrossRef]
- Maggi, M.; Del Carlo, P.; Fantoni, G.; Giannini, S.; Torrisi, C.; Casparis, D.; Massi, G.; Serio, M. Human myometrium during pregnancy contains and responds to V1 vasopressin receptors as well as oxytocin receptors. J. Clin. Endocrinol. Metab. 1990, 70, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Peacock, S.O.; Lo, C.H.; Heidman, L.M.; Rice, M.A.; Fahrenholtz, C.D.; Greene, A.M.; Magani, F.; Copello, V.A.; Martinez, M.J.; et al. Arginine vasopressin receptor 1a is a therapeutic target for castration-resistant prostate cancer. Sci. Transl. Med. 2019, 11, eaaw4636. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.H.; Holmes, C.L.; Wang, Y.; Roberts, H.; Walley, K.R. Vasopressin decreases sepsis-induced pulmonary inflammation through the V2R. Resuscitation 2008, 79, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Tamma, R.; Sun, L.; Cuscito, C.; Lu, P.; Corcelli, M.; Li, J.; Colaianni, G.; Moonga, S.S.; Di Benedetto, A.; Grano, M.; et al. Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia. Proc. Natl. Acad. Sci. USA 2013, 110, 18644–18649. [Google Scholar] [CrossRef] [Green Version]
- Phillips, P.A.; Abrahams, J.M.; Kelly, J.M.; Mooser, V.; Trinder, D.; Johnston, C.I. Localization of vasopressin binding sites in rat tissues using specific V1 and V2 selective ligands. Endocrinology 1990, 126, 1478–1484. [Google Scholar] [CrossRef]
- Zimmermann-Peruzatto, J.M.; Lazzari, V.M.; de Moura, A.C.; Almeida, S.; Giovenardi, M. Examining the role of vasopressin in the modulation of parental and sexual behaviors. Front. Psychiatry 2015, 6, 130. [Google Scholar] [CrossRef] [Green Version]
- Thibonnier, M.; Graves, M.K.; Wagner, M.S.; Auzan, C.; Clauser, E.; Willard, H.F. Structure, sequence, expression, and chromosomal localization of the human v1avasopressin receptor gene. Genomics 1996, 31, 327–334. [Google Scholar] [CrossRef]
- Wasilewski, M.A.; Myers, V.D.; Recchia, F.A.; Feldman, A.M.; Tilley, D.G. Arginine vasopressin receptor signaling and functional outcomes in heart failure. Cell. Signal. 2016, 28, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Birnbaumer, M. Vasopressin receptors. Trends Endocrinol. Metab. 2000, 11, 406–410. [Google Scholar] [CrossRef]
- Machida, K.; Wakamatsu, S.; Izumi, Y.; Yosifovska, T.; Matsuzaki, T.; Nakayama, Y.; Kohda, Y.; Inoue, T.; Saito, H.; Tomita, K.; et al. Downregulation of the V2 vasopressin receptor in dehydration: Mechanisms and role of renal prostaglandin synthesis. Am. J. Phys. Renal Phys. 2007, 292, F1274–F1282. [Google Scholar] [CrossRef]
- Sebti, Y.; Rabbani, M.; Sadeghi, H.; Sadeghi, H.M.M.; Sardari, S.; Ghahremani, M.H.; Innamorati, G. Effect of mutations in putative hormone binding sites on V2 vasopressin receptor function. Res. Pharm. Sci. 2015, 10, 259–267. [Google Scholar] [PubMed]
- Hoffert, J.D.; Chou, C.L.; Fenton, R.A.; Knepper, M.A. Calmodulin is required for vasopressin-stimulated increase in cyclic AMP production in inner medullary collecting duct. J. Biol. Chem. 2005, 280, 13624–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robben, J.H.; Knoers, N.V.; Deen, P.M. Regulation of the vasopressin V2 receptor by vasopressin in polarized renal collecting duct cells. Mol. Biol. Cell 2004, 15, 5693–5699. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Gupta, A. Aquaporins: The renal water channels. Indian J. Nephrol. 2008, 18, 95–100. [Google Scholar] [CrossRef]
- Park, E.J.; Kwon, T.H. A minireview on vasopressin-regulated aquaporin-2 in kidney collecting duct cells. Electrolyte Blood Press. 2015, 13, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Olesen, E.T.; Fenton, R.A. Aquaporin-2 membrane targeting: Still a conundrum. Am. J. Physiol. Renal Physiol. 2017, 312, F744–F747. [Google Scholar] [CrossRef] [Green Version]
- Friberg, M.A.; Spiess, M.; Rutishauser, J. Degradation of wild-type vasopressin precursor and pathogenic mutants by the proteasome. J. Biol. Chem. 2004, 279, 19441–19447. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.; Somlo, D.R.M.; Kim, G.H.; Prescianotto-Baschong, C.; Sun, S.; Beuret, N.; Long, Q.; Rutishauser, J.; Arvan, P.; Spiess, M.; et al. ER-associated degradation is required for vasopressin prohormone processing and systemic water homeostasis. J. Clin. Investig. 2017, 127, 3897–3912. [Google Scholar] [CrossRef] [Green Version]
- Gubbi, S.; Hannah-Shmouni, F.; Verbalis, J.G.; Koch, C.A. Hypophysitis: An update on the novel forms, diagnosis and management of disorders of pituitary inflammation. Best Prac. Res. Clin. Endocrinol. Metab. 2019, 33, 101371. [Google Scholar] [CrossRef]
- Bichet, D. Vasopressin receptors in health and disease. Kidney Int. 1996, 49, 1706–1711. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Dalal, S.; Singh, K. Osteopontin: At the cross-roads of myocyte survival and myocardial function. Life Sci. 2014, 118, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazari, A.; Sadr, S.S.; Faghihi, M.; Imani, A.; Moghimian, M. The cardioprotective effect of different doses of vasopressin (AVP) against ischemia-reperfusion injuries in the anesthetized rat heart. Peptides 2011, 32, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Tilley, D.G.; Myers, V.D.; Tsai, E.J.; Feldman, A.M. Increased vasopressin 1a receptor expression in failing human heart. J. Am. Coll. Cardiol. 2014, 63, 375–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanoue, A.; Ito, S.; Honda, K.; Oshikawa, S.; Kitagawa, Y.; Koshimizu, T.A.; Mori, T.; Tsujimoto, G. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J. Clin. Investig. 2004, 113, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, E.L.; Caldwell, H.K. The vasopressin 1b receptor and the neural regulation of social behavior. Horm. Behav. 2012, 61, 277–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, S.R.; Moffett, C.; Thomas, K.G.; Irwin, N.; Flatt, P.R. Vasopressin receptors in islets enhance glucose tolerance, pancreatic beta-cell secretory function, proliferation and survival. Biochimie 2019, 158, 191–198. [Google Scholar] [CrossRef]
- Taveau, C.; Chollet, C.; Waeckel, L.; Desposito, D.; Bichet, D.G.; Arthus, M.F.; Magnan, C.; Philippe, E.; Paradis, V.; Foufelle, F.; et al. Vasopressin and hydration play a major role in the development of glucose intolerance and hepatic steatosis in obese rats. Diabetologia 2015, 58, 1081–1090. [Google Scholar] [CrossRef] [Green Version]
- Taveau, C.; Chollet, C.; Bichet, D.G.; Velho, G.; Guillon, G.; Corbani, M.; Roussel, R.; Bankir, L.; Melander, O.; Bouby, N. Acute and chronic hyperglycemic effects of vasopressin in normal rats: Involvement of V1a receptors. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E127–E135. [Google Scholar] [CrossRef]
- Tashima, Y.; Kohda, Y.; Nonoguchi, H.; Ikebe, M.; Machida, K.; Star, R.A.; Tomita, K. Intranephron localization and regulation of the V1a vasopressin receptor during chronic metabolic acidosis and dehydration in rats. Pflugers Arch. 2001, 442, 652–661. [Google Scholar] [CrossRef]
- Martin, N.P.; Lefkowitz, R.J.; Shenoy, S.K. Regulation of V2 vasopressin receptor degradation by agonist-promoted ubiquitination. J. Biol. Chem. 2003, 278, 45954–45959. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Sharma, N.; Zheng, W.; Ji, H.; Tam, H.; Wu, X.; Manigrasso, M.B.; Sandberg, K.; Verbalis, J.G. Sex differences in vasopressin V2 receptor expression and vasopressin-induced antidiuresis. Am. J. Physiol. Renal Physiol. 2011, 300, F433–F440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncharova, N.D. Stress responsiveness of the hypothalamic-pituitary-adrenal axis: Age-related features of the vasopressinergic regulation. Front. Endocrinol. (Lausanne) 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiwazaki, A.; Fujiwara, Y.; Tsuchiya, H.; Sakai, N.; Shibata, K.; Koshimizu, T.A. Subcellular localization and internalization of the vasopressin V1B receptor. Eur. J. Pharmacol. 2015, 765, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Birnbaumer, M.; Antaramian, A.; Themmen, A.P.; Gilbert, S. Desensitization of the human V2 vasopressin receptor. Homologous effects in the absence of heterologous desensitization. J. Biol. Chem. 1992, 267, 11783–11788. [Google Scholar] [CrossRef]
- Nathanson, M.H.; Burgstahler, A.D.; Orloff, J.J.; Mani, A.; Moyer, M.S. Mechanism of desensitization of the cloned vasopressin V1a receptor expressed in Xenopus oocytes. Am. J. Physiol. Cell Physiol. 1994, 267, C94–C103. [Google Scholar] [CrossRef] [PubMed]
- Ancellin, N.; Morel, A. Homologous and heterologous acute desensitization of vasopressin V1a receptor in Xenopus oocytes. Cell. Signal. 1998, 10, 217–223. [Google Scholar] [CrossRef]
- Innamorati, G.; Sadeghi, H.M.; Tran, N.T.; Birnbaumer, M. A serine cluster prevents recycling of the V2 vasopressin receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 2222–2226. [Google Scholar] [CrossRef] [Green Version]
- Innamorati, G.; Sadeghi, H.M.; Tran, N.T.; Birnbaumer, M. Transient phosphorylation of the V1a vasopressin receptor. J. Biol. Chem. 1998, 273, 7155–7166. [Google Scholar] [CrossRef] [Green Version]
- Innamorati, G.; Le Gouill, C.; Balamotis, M.; Birnbaumer, M. The long and the short cycle: Alternative intracellular routes for trafficking of G-protein-coupled receptors. J. Biol. Chem. 2001, 276, 13096–13103. [Google Scholar] [CrossRef] [Green Version]
- Ancellin, N.; Preisser, L.; Le Maout, S.; Barbado, M.; Creminon, C.; Corman, B.; Morel, A. Homologous and heterologous phosphorylation of the vasopressin V1a receptor. Cell. Signal. 1999, 11, 743–751. [Google Scholar] [CrossRef]
- Bichet, D.G. V2R mutations and nephrogenic diabetes insipidus. Prog. Mol. Biol. Transl. Sci. 2009, 89, 15–29. [Google Scholar] [PubMed]
- Bockenhauer, D.; Bichet, D.G. Nephrogenic diabetes insipidus. Curr. Opin. Pediatr. 2017, 29, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bichet, D.G. Genetics in Endocrinology Pathophysiology, diagnosis and treatment of familial nephrogenic diabetes insipidus. Eur. J. Endocrinol. 2020, 183, R29–R40. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers. 2018, 4, 50. [Google Scholar] [CrossRef]
- Harris, P.C.; Torres, V.E. Polycystic kidney disease. Annu. Rev. Med. 2009, 60, 321–337. [Google Scholar] [CrossRef]
- Bergmann, C.; Senderek, J.; Küpper, F.; Schneider, F.; Dornia, C.; Windelen, E.; Eggermann, T.; Rudnik-Schoneborn, S.; Kirfel, J.; Furu, L.; et al. PKHD1 mutations in autosomal recessive polycystic kidney disease (ARPKD). Hum. Mut. 2004, 23, 453–463. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Ward, C.J.; Harris, P.C.; Torres, V.E. Vasopressin directly regulates cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 2008, 19, 102–108. [Google Scholar] [CrossRef]
- Hughes, J.; Ward, C.J.; Peral, B.; Aspinwall, R.; Clark, K.; San Millan, J.L.; Gamble, V.; Harris, P.C. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 1995, 10, 151–160. [Google Scholar] [CrossRef]
- Hanaoka, K.; Qian, F.; Boletta, A.; Bhunia, A.K.; Piontek, K.; Tsiokas, L.; Sukhatme, V.P.; Guggino, W.B.; Germino, G.G. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 2000, 408, 990–994. [Google Scholar] [CrossRef]
- Bai, C.X.; Giamarchi, A.; Rodat-Despoix, L.; Padilla, F.; Downs, T.; Tsiokas, L.; Delmas, P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep. 2008, 9, 472–479. [Google Scholar] [CrossRef] [Green Version]
- Onuchic, L.F.; Furu, L.; Nagasawa, Y.; Hou, X.; Eggermann, T.; Ren, Z.; Bergmann, C.; Senderek, J.; Esquivel, E.; Zeltner, R.; et al. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am. J. Hum. Genet. 2002, 70, 1305–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, C.J.; Hogan, M.C.; Rossetti, S.; Walker, D.; Sneddon, T.; Wang, X.; Kubly, V.; Cunningham, J.M.; Bacallao, R.; Ishibashi, M.; et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat. Genet. 2002, 30, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Chen, Y.; Yi, Y.; Tsuchiya, K.; Moeckel, G.; Cheung, J.; Liang, D.; Tham, K.; Xu, X.; Chen, X.Z.; et al. A novel gene encoding a TIG multiple domain protein is a positional candidate for autosomal recessive polycystic kidney disease. Genomics 2002, 80, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Fu, Y.; Hui, K.; Moeckel, G.; Mai, W.; Li, C.; Liang, D.; Zhao, P.; Ma, J.; Chen, X.Z. Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J. Am. Soc. Nephrol. 2008, 19, 455–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Koch, G.; Ouyang, J.; McQuade, R.D.; Blais, J.D.; Czerwiec, F.S.; et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2017, 377, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Harris, P.C. Mechanisms of disease: Autosomal dominant and recessive polycystic kidney diseases. Nat. Rev. Nephrol. 2006, 2, 40–55. [Google Scholar] [CrossRef]
- Devuyst, O.; Torres, V.E. Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 2013, 22, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Chebib, F.T.; Sussman, C.R.; Wang, X.; Harris, P.C.; Torres, V.E. Vasopressin and disruption of calcium signalling in polycystic kidney disease. Nat. Rev. Nephrol. 2015, 11, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Seeger-Nukpezah, T.; Geynisman, D.M.; Nikonova, A.S.; Benzing, T.; Golemis, E.A. The hallmarks of cancer: Relevance to the pathogenesis of polycystic kidney disease. Nat. Rev. Nephrol. 2015, 11, 515–534. [Google Scholar] [CrossRef] [Green Version]
- Nagao, S.; Nishii, K.; Katsuyama, M.; Kurahashi, H.; Marunouchi, T.; Takahashi, H.; Wallace, D.P. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J. Am. Soc. Nephrol. 2006, 17, 2220–2227. [Google Scholar] [CrossRef] [Green Version]
- Reif, G.A.; Yamaguchi, T.; Nivens, E.; Fujiki, H.; Pinto, C.S.; Wallace, D.P. Tolvaptan inhibits ERK-dependent cell proliferation, Cl− secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am. J. Physiol. Renal Physiol. 2011, 301, F1005–F1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinschen, M.M.; Schermer, B.; Benzing, T. Vasopressin-2 receptor signaling and autosomal dominant polycystic kidney disease: From bench to bedside and back again. J. Am. Soc. Nephrol. 2014, 25, 1140–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Yu, S. New insights into the molecular mechanisms targeting tubular channels/transporters in PKD development. Kidney Dis. 2016, 2, 128–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, C.S.; Reif, G.A.; Nivens, E.; White, C.; Wallace, D.P. Calmodulin-sensitive adenylyl cyclases mediate AVP-dependent cAMP production and Cl− secretion by human autosomal dominant polycystic kidney cells. Am. J. Physiol. Renal Physiol. 2012, 303, F1412–F1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belibi, F.A.; Reif, G.; Wallace, D.P.; Yamaguchi, T.; Olsen, L.; Li, H. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int. 2004, 66, 964–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gradilone, S.A.; Masyuk, T.V.; Huang, B.Q.; Banales, J.M.; Lehmann, G.L.; Radtke, B.N.; Stroope, A.; Masyuk, A.I.; Splinter, P.L.; LaRusso, N.F. Activation of Trpv4 reduces the hyperproliferative phenotype of cystic cholangiocytes from an animal model of ARPKD. Gastroenterology 2010, 139, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Juul, K.V.; Bichet, D.G.; Nielsen, S.; Nørgaard, J.P. The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am. J. Physiol. Renal Physiol. 2014, 306, F931–F940. [Google Scholar] [CrossRef]
- Ishikawa, I.; Maeda, K.; Nakai, S.; Kawaguchi, Y. Gender difference in the mean age at the induction of hemodialysis in patients with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2000, 35, 1072–1075. [Google Scholar] [CrossRef]
- Bae, K.T.; Zhou, W.; Shen, C.; Landsittel, D.P.; Wu, Z.; Tao, C.; Chapman, A.B.; Torres, V.E.; Lu, A.S.L.; Mrug, M.; et al. Growth pattern of kidney cyst number and volume in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2019, 14, 823–833. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar]
- Ishikawa, S. Arginine vasopressin in heart failure. Circ. J. 2014, 78, 2161–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Lu, G.; Tang, K.; Li, Q.; Gao, X. The secretion patterns and roles of cardiac and circulating arginine vasopressin during the development of heart failure. Neuropeptides 2015, 51, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hackett, P.D.; DeMarco, A.C.; Feng, C.; Stair, S.; Haroon, E.; Ditzen, B.; Pagnoni, G.; Rilling, J.K. Effects of oxytocin and vasopressin on the neural response to unreciprocated cooperation within brain regions involved in stress and anxiety in men and women. Brain Imaging Behav. 2016, 10, 581–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, J.M.; Zeballos, G.A.; Woolf, P.K.; Dweck, H.S.; Gewitz, M.H. Variable arginine vasopressin levels in neonatal congestive heart failure. J. Am. Coll. Cardiol. 1988, 11, 645–650. [Google Scholar] [CrossRef] [Green Version]
- Szmydynger-Chodobska, J.; Zink, B.J.; Chodobski, A. Multiple sites of vasopressin synthesis in the injured brain. J. Cereb. Blood Flow Metab. 2011, 31, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Hiroyama, M.; Wang, S.; Aoyagi, T.; Oikawa, R.; Sanbe, A.; Takeo, S.; Tanoue, A. Vasopressin promotes cardiomyocyte hypertrophy via vasopressin V1A receptor in neonatal mice. Eur. J. Pharmacol. 2007, 559, 89–97. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, L.; Zheng, Q.; Li, X. Effects of arginine vasopressin on growth of rat cardiac fibroblasts: Role of V1 receptor. J. Cardiovasc. Pharmacol. 2003, 42, 132–135. [Google Scholar] [CrossRef]
- Czarzasta, K.; Wojno, O.; Zera, T.; Puchalska, L.; Dobruch, J.; Cudnoch-Jedrzejewska, A. The influence of post-infarct heart failure and high fat diet on the expression of apelin APJ and vasopressin V1a and V1b receptors. Neuropeptides 2019, 78, 101975. [Google Scholar] [CrossRef]
- Goldsmith, S.R.; Gheorghiade, M. Vasopressin antagonism in heart failure. J. Am. Coll. Cardiol. 2005, 46, 1786–1791. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chan, T.O.; Myers, V.; Chowdhury, I.; Zhang, X.Q.; Song, J.; Zhang, J.; Andrel, J.; Funakoshi, H.; Robbins, J.; et al. Controlled and cardiac-restricted overexpression of the arginine vasopressin V1a receptor causes reversible left ventricular dysfunction through Gαq-mediated cell signaling. Circulation 2011, 124, 572–581. [Google Scholar] [CrossRef] [Green Version]
- Tilley, D.G.; Zhu, W.; Myers, V.D.; Barr, L.A.; Gao, E.; Li, X.; Song, J.; Carter, R.L.; Makarewich, C.A.; Yu, D.; et al. β-Adrenergic receptor-mediated cardiac contractility is inhibited via vasopressin type 1A-receptor-dependent signaling. Circulation 2014, 130, 1800–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Xu, F.; Zhang, L.; Wang, X.; Wang, Y.; Woo, A.Y.H.; Zhu, W. GRK2/β-arrestin mediates arginine vasopressin-induced cardiac fibroblast proliferation. Clin. Exp. Pharmacol. Physiol. 2017, 44, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Abraham, W.T. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 1999, 341, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Rossi, J.S.; Cotts, W.; Shin, D.D.; Hellkamp, A.S.; Pina, I.L.; Fonarow, G.C.; DeMarco, T.; Pauly, D.F.; Rogers, J.; et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch. Intern. Med. 2007, 167, 1998–2005. [Google Scholar] [CrossRef]
- Xu, Y.J.; Gopalakrishnan, V. Vasopressin increases cytosolic free [Ca2+] in the neonatal rat cardiomyocyte. Circ. Res. 1991, 69, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Martin, P.; Ohara, M.; St John, J.; Pattison, T.; Meng, X.; Morris, K.; Kim, J.K.; Schrier, R.W. Upregulation of aquaporin-2 water channel expression in chronic heart failure rat. J. Clin. Investigat. 1997, 99, 1500–1505. [Google Scholar] [CrossRef] [Green Version]
- Brønd, L.; Müllertz, K.M.; Torp, M.; Nielsen, J.; Graebe, M.; Hadrup, N.; Jonassen, T.E.N. Congestive heart failure in rats is associated with increased collecting duct vasopressin sensitivity and vasopressin type 2 receptor re-externalization. Am. J. Physiol. Renal Physiol. 2013, 305, F1547–F1554. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.S. Oxytocin pathways and the evolution of human behavior. Annu. Rev. Psychol. 2014, 65, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Veenema, A.H.; Neumann, I.D. Central vasopressin and oxytocin release: Regulation of complex social behaviours. Prog. Brain Res. 2008, 170, 261–276. [Google Scholar]
- Huber, D.; Veinante, P.; Stoop, R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 2005, 308, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, J.; Reisine, T.D. Stress hormones: Their interaction and regulation. Science 1984, 224, 452–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planas, B.; Kolb, P.E.; Raskind, M.A.; Miller, M.A. Sex difference in coexpression by galanin neurons accounts for sexual dimorphism of vasopressin in the bed nucleus of the stria terminalis. Endocrinology 1995, 136, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.; Bass, A. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev. 2001, 35, 246–265. [Google Scholar] [CrossRef]
- Quintana, L.; Pouso, P.; Fabbiani, G.; Macadar, O. A central pacemaker that underlies the production of seasonal and sexually dimorphic social signals: Anatomical and electrophysiological aspects. J. Comp. Physiol. A. Neuroethol. Sens. Neural Behav. Physiol. 2011, 197, 75–88. [Google Scholar] [CrossRef]
- Buijs, R.M.; De Vries, G.J.; Van Leeuwen, F.W.; Swaab, D.F. Vasopressin and oxytocin: Distribution and putative functions in the brain. Prog. Brain Res. 1983, 60, 115–122. [Google Scholar]
- Antoni, F.A. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front. Neuroendocrinol. 1993, 14, 76–122. [Google Scholar] [CrossRef]
- Leng, G.; Brown, C.H.; Russell, J.A. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog. Neurobiol. 1999, 57, 625–655. [Google Scholar] [CrossRef]
- Landgraf, R.; Neumann, I.D. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004, 25, 150–176. [Google Scholar] [CrossRef]
- Armstrong, W.E. Hypothalamic supraoptic and paraventricular nuclei. In The Rat Nervous System, 4th ed.; Paxinos, G., Ed.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 295–314. [Google Scholar]
- Sofroniew, M.V. Vasopressin and oxytocin in the mammalian brain and spinal cord. Front. Neuroendocrinol. 1983, 40, 1–23. [Google Scholar] [CrossRef]
- Albers, H.E. Species, sex and individual differences in the vasotocin/vasopressin system: Relationship to neurochemical signaling in the social behavior neural network. Front. Neuroendocrinol. 2015, 36, 49–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacker, D.; Ludwig, M. The role of vasopressin in olfactory and visual processing. Cell Tissue Res. 2019, 375, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Leng, G. Dendritic neuropeptide release and peptide dependent behaviours. Nat. Rev. Neurosci. 2006, 7, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Stern, J. Multiple signalling modalities mediated by dendritic exocytosis of oxytocin and vasopressin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motoki, K.; Sugiura, M.; Takeuchi, H.; Kotozaki, Y.; Nakagawa, S.; Yokoyama, R.; Kawashima, R. Are plasma oxytocin and vasopressin levels reflective of amygdala activation during the processing of negative emotions? A preliminary study. Front. Psychol. 2016, 7, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sausville, E.; Carney, D.; Battey, J. The human vasopressin gene is linked to the oxytocin gene and is selectively expressed in a cultured lung cancer cell line. J. Biol. Chem. 1985, 260, 10236–10241. [Google Scholar] [CrossRef]
- Acher, R.; Chauvet, J.; Chauvet, M.T. Man and the chimaera. Selective versus neutral oxytocin evolution. Adv. Exp. Med. Biol. 1995, 395, 615–627. [Google Scholar]
- Legros, J.J. Inhibitory effect of oxytocin on corticotrope function in humans: Are vasopressin and oxytocin ying-yang neurohormones? Psychoneuroendocrinology 2001, 26, 649–655. [Google Scholar] [CrossRef]
- Carter, C.S.; Grippo, A.J.; Pournajafi-Nazarloo, H.; Ruscio, M.G.; Porges, S.W. Oxytocin, vasopressin and sociality. Prog. Brain Res. 2008, 170, 331–336. [Google Scholar]
- Neumann, I.D.; Landgraf, R. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012, 35, 649–659. [Google Scholar] [CrossRef]
- Stoop, R. Neuromodulation by oxytocin and vasopressin. Neuron 2012, 76, 142–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferris, C. Role of vasopressin in aggressive and dominant/subordinate behaviors. Ann. N. Y. Acad. Sci. 1992, 652, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Moore, F.L.; Lowry, C.A. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp. Biochem. Physiol. 1998, 119, 251–260. [Google Scholar] [CrossRef]
- Newman, S.W. The medial extended amygdala in male reproductive behaviour. A node in the mammalian social reproductive behaviour. Ann. N. Y. Acad. Sci. 1999, 877, 242–257. [Google Scholar] [CrossRef]
- Engelmann, M.; Wotjak, C.T.; Ebner, K.; Landgraf, R. Behavioural impact of intraseptally released vasopressin and oxytocin in rats. Exp. Physiol. 2000, 85, 125S–130S. [Google Scholar] [CrossRef]
- Gilligan, P.; Brenner, S.; Venkatesh, B. Neurone-specific expression and regulation of the puffer fish isotocin and vasotocin genes in transgenic mice. J. Neuroendocrinol. 2003, 15, 1027–1036. [Google Scholar] [CrossRef]
- Goodson, J.L. The vertebrate social behavior network: Evolutionary themes and variations. Horm. Behav. 2005, 48, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Storm, E.E.; Tecott, L.H. Social circuits: Peptidergic regulation of mammalian social behavior. Neuron 2005, 47, 483–486. [Google Scholar] [CrossRef] [Green Version]
- De Vries, G.J.; Panzica, G.C. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: Different mechanisms, similar endpoints. Neuroscience 2006, 138, 947–955. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, Z.R.; Young, L.J. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 2008, 322, 900–904. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, H.K.; Aulino, E.A.; Rodriguez, K.M.; Witchey, S.K.; Yaw, A.M. Social context, stress, neuropsychiatric disorders, and the vasopressin 1b receptor. Front. Neurosci. 2017, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Griebel, G.; Holsboer, F. Neuropeptide receptor ligands as drugs for psychiatric diseases: The end of the beginning? Nat. Rev. Drug. Discov. 2012, 11, 462–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, N.; Shang, S.; Su, Y. The arginine vasopressin V1b receptor gene and prosociality: Mediation role of emotional empathy. PsyCh J. 2015, 4, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Roper, J.; O’Carroll, A.M.; Young, W.; Lolait, S. The vasopressin Avpr1b receptor: Molecular and pharmacological studies. Stress 2011, 14, 98–115. [Google Scholar] [CrossRef]
- Barsegyan, A.; Atsak, P.; Hornberger, W.B.; Jacobson, P.B.; Van Gaalen, M.M.; Roozendaal, B. The vasopressin 1b receptor antagonist A-988315 blocks stress effects on the retrieval of object-recognition memory. Neuropsychopharmacology 2015, 40, 1979–1989. [Google Scholar] [CrossRef] [Green Version]
- Wersinger, S.R.; Caldwell, H.K.; Christiansen, M.; Young, W.S. Disruption of the vasopressin 1b receptor gene impairs the attack component of aggressive behavior in mice. Genes Brain Behav. 2007, 6, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Bosch, O.J. Maternal aggression in rodents: Brain oxytocin and vasopressin mediate pup defence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20130085. [Google Scholar] [CrossRef] [Green Version]
- Wersinger, S.R.; Ginns, E.I.; O’Carroll, A.M.; Lolait, S.J.; Young III, W.S. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol. Psychiatry 2002, 7, 975–984. [Google Scholar] [CrossRef] [Green Version]
- Wersinger, S.R.; Kelliherb, K.R.; Zufallb, F.; Lolait, S.J.; O’Carroll, A.M.; Young, W.S., III. Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Horm. Behav. 2004, 46, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, J.N.; Young, L.J.; Insel, T.R. The neuroendocrine basis of social recognition. Front. Neuroendocrinol. 2002, 23, 200–224. [Google Scholar] [CrossRef] [Green Version]
- Bales, K.L.; Kim, A.J.; Lewis-Reese, A.D.; Carter, C.S. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm. Behav. 2004, 45, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Bielsky, I.; Bao-Hu, S.; Szegda, K.; Westphal, H.; Young, L. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 2004, 29, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Albers, H.E. The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Horm. Behav. 2012, 61, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Stemmelin, J.; Lukovic, L.; Salome, N.; Griebel, G. Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V1b receptor antagonist, SSR149415. Neuropsychopharmacology 2005, 30, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Frazier, C.R.; Trainor, B.C.; Cravens, C.J.; Whitney, T.K.; Marler, C.A. Paternal behavior influences development of aggression and vasopressin expression in male California mouse offspring. Horm. Behav. 2006, 50, 699–707. [Google Scholar] [CrossRef]
- Clipperton-Allen, A.E.; Lee, A.W.; Reyes, A.; Devidze, N.; Phan, A.; Pfaff, D.W.; Choleris, E. Oxytocin, vasopressin and estrogen receptor gene expression in relation to social recognition in female mice. Physiol. Behav. 2012, 105, 915–924. [Google Scholar] [CrossRef] [Green Version]
- Wacker, D.W.; Engelmann, M.; Tobin, V.A.; Meddle, S.L.; Ludwig, M. Vasopressin and social odor processing in the olfactory bulb and anterior olfactory nucleus. Ann. N. Y. Acad. Sci. 2011, 1220, 106–116. [Google Scholar] [CrossRef]
- Tobin, V.A.; Hashimoto, H.; Wacker, D.W.; Takayanagi, Y.; Langnaese, K.; Caquineau, C.; Noack, J.; Landgraf, R.; Onaka, T.; Leng, G.; et al. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature 2010, 464, 413–417. [Google Scholar] [CrossRef]
- Pineda, R.; Plaisier, F.; Millar, R.P.; Ludwig, M. Amygdala kisspeptin neurons: Putative mediators of olfactory control of the gonadotropic axis. Neuroendocrinology 2017, 104, 223–238. [Google Scholar] [CrossRef] [Green Version]
- De Vries, G.J.; Buijs, R.M. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983, 273, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Goodson, J.L. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla). Horm. Behav. 1998, 34, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.L.; Adkins-Regan, E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata). J. Neuroendocrinol. 1999, 11, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koolhaas, J.M.; Everts, H.; de Ruiter, A.J.; de Boer, S.F.; Bohus, B. Coping with stress in rats and mice: Differential peptidergic modulation of the amygdala-lateral septum complex. Prog. Brain Res. 1998, 119, 437–448. [Google Scholar] [PubMed]
- Pedersen, C.A.; Ascher, J.A.; Monroe, Y.L.; Prange, A.J., Jr. Oxytocin induces maternal behavior in virgin female rats. Science 1982, 216, 648–650. [Google Scholar] [CrossRef]
- Pedersen, C.A.; Caldwell, J.D.; Johnson, M.F.; Fort, S.A.; Prange, A.J., Jr. Oxytocin antiserum delays onset of ovarian steroid-induced maternal behavior. Neuropeptide 1985, 6, 175–182. [Google Scholar] [CrossRef]
- Pedersen, C.A.; Caldwell, J.D.; Walker, C.; Ayers, G.; Mason, G.A. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav. Neurosci. 1994, 108, 1163–1171. [Google Scholar] [CrossRef]
- Wang, Z.; Ferris, C.F.; De Vries, G.J. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster). Proc. Natl. Acad. Sci. USA 1994, 91, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Argiolas, A.; Melis, M.R. Neuropeptides and central control of sexual behaviour from the past to the present: A review. Prog. Neurobiol. 2013, 108, 80–107. [Google Scholar] [CrossRef]
- De Vries, G.J.; Al-Shamma, H.A. Sex differences in hormonal responses of vasopressin pathways in the rat brain. J. Neurobiol. 1990, 21, 686–693. [Google Scholar] [CrossRef]
- Urban, J.H.; Miller, M.A.; Dorsa, D.M. Dexamethasone-induced suppression of vasopressin gene-expression in the bed nucleus of the stria terminalis and medial amygdala is mediated by changes in testosterone. Endocrinology 1991, 129, 109–116. [Google Scholar] [CrossRef]
- De Vries, G.J.; Wang, Z.; Bullock, N.A.; Numan, S. Sex differences in the effects of testosterone and its metabolites on vasopressin messenger RNA levels in the bed nucleus of the stria terminalis of rats. J. Neurosci. 1994, 14, 1789–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delville, Y.; Mansour, K.M.; Ferris, C.F. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol. Behav. 1996, 60, 25–29. [Google Scholar] [CrossRef]
- Lebow, M.; Chen, A. Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 2016, 21, 450–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vries, G.J.; Buijs, R.M.; Swaab, D.F. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain—presence of a sex difference in the lateral septum. Brain Res. 1981, 218, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Fink, G.; Rosie, R.; Sheword, W.J.; Thomson, E.; Wilson, H. Steroid control of central neuronal interactions and function. J. Steroid Biochem. Mol. Biol. 1991, 40, 123–132. [Google Scholar] [CrossRef]
- De Vries, G.J.; Södersten, P. Sex differences in the brain: The relation between structure and function. Horm. Behav. 2009, 55, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Villalon, A.L.; Garcia, J.L.; Fernandez, N.; Monge, L.; Gomez, B.; Dieguez, G. Regional differences in the arterial response to vasopressin: Role of endothelial nitric oxide. Br. J. Pharmacol. 1996, 118, 1848–1854. [Google Scholar] [CrossRef]
- Rosen, G.J.; De Vries, G.J.; Goldman, S.L.; Goldman, B.D.; Forger, N.G. Distribution of vasopressin in the brain of the eusocial naked mole-rat. J. Comp. Neurol. 2007, 500, 1093–1105. [Google Scholar] [CrossRef]
- Qiao, X.; Yan, Y.; Wu, R.; Tai, F.; Hao, P.; Cao, Y.; Wang, J. Sociality and oxytocin and vasopressin in the brain of male and female dominant and subordinate mandarin voles. J. Comp. Physiol. A, Neuroethol. Sens. Neural Behav. Physiol. 2014, 200, 149–159. [Google Scholar] [CrossRef]
- Grundwald, N.J.; Benítez, D.P.; Brunton, P.J. Sex-dependent effects of prenatal stress on social memory in rats: A role for differential expression of central vasopressin-1a receptors. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef]
- Hermes, M.; Buijs, R.; Masson-Pévet, M.; Pevet, P. Seasonal changes in vasopressin in the brain of the garden dormouse (Eliomys quercinus L.). J. Comp. Neurol. 1990, 293, 340–346. [Google Scholar] [CrossRef]
- Goodson, J.L.; Wilson, L.C.; Schrock, S.E. To flock or fight: Neurochemical signatures of divergent life histories in sparrows. Proc. Natl. Acad. Sci. USA 2012, 109, 10685–10692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamshad, M.; Novak, M.A.; Vries, G.J. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster and meadow voles, Microtus pennsylvanicus. J. Neuroendocrinol. 1993, 5, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Kozorovitskiy, Y.; Hughes, M.; Lee, K.; Gould, E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat. Neurosci. 2006, 9, 1094–1095. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.G.; Franssen, C.L.; Bardi, M.; Hampton, J.E.; Hainley, L.; Karsner, S.; Tu, E.B.; Hyer, M.M.; Crockett, A.; Baranova, A.; et al. Characteristic neurobiological patterns differentiate paternal responsiveness in two Peromyscus species. Brain Behav. Evol. 2011, 77, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Perea-Rodriguez, J.; Takahashi, E.; Amador, T.; Hao, R.; Saltzman, W.; Trainor, B. Effects of reproductive experience on central expression of progesterone, oestrogen α, oxytocin and vasopressin receptor mRNA in male California mice (Peromyscus californicus). J. Neuroendocrinol. 2015, 27, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, T.; Allchorne, A.J.; Zhang, M.; Tsuji, C.; Tobin, V.A.; Pineda, R.; Raftogianni, A.; Stern, J.E.; Grinevich, V.; Leng, G.; et al. Vasopressin casts light on the suprachiasmatic nucleus. J. Physiol. 2017, 595, 3497–3514. [Google Scholar] [CrossRef] [PubMed]
- Gizowski, C.; Zaelzer, C.; Bourque, C. Clock-driven vasopressin neurotransmission mediates anticipatory thirst prior to sleep. Nature 2016, 537, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Gizowski, C.; Zaelzer, C.; Bourque, C.W. Activation of organum vasculosum neurones and water intake in mice by vasopressin neurones in the suprachiasmatic nucleus. J. Neuroendocrinol. 2018, 30, e12577. [Google Scholar] [CrossRef] [PubMed]
- Ebstein, R.P.; Israel, S.; Lerer, E.; Uzefovsky, F.; Shalev, I.; Gritsenko, I.; Riebold, M.; Salomon, S.; Yirmiya, N. Arginine vasopressin and oxytocin modulate human social behavior. Ann. N. Y. Acad. Sci. 2009, 1167, 87–102. [Google Scholar] [CrossRef]
- Crockford, C.; Deschner, T.; Ziegler, T.E.; Wittig, R.M. Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: A review. Front. Behav. Neurosci. 2014, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.; Monakhov, M.; Pratt, M.; Ebstein, R.P. Oxytocin pathway genes: Evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol. Psychiatry 2016, 79, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, P.; Kalisch, R.; Singer, T.; Dolan, R.J. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J. Neurosci. 2008, 28, 6607–6615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheele, D.; Striepens, N.; Gunturkun, O.; Deutschlander, S.; Maier, W.; Kendrick, K.M.; Hurlemann, R. Oxytocin modulates social distance between males and females. J. Neurosci. 2012, 32, 16074–16079. [Google Scholar] [CrossRef] [PubMed]
- Preckel, K.; Scheele, D.; Kendrick, K.M.; Maier, W.; Hurlemann, R. Oxytocin facilitates social approach behavior in women. Front. Behav. Neurosci. 2014, 8, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, L.B.; Walum, H.; Inoue, K.; Eyrich, N.W.; Young, L.J. Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biol. Psychiatry 2016, 80, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunnlieb, C.; Münte, T.F.; Tempelmann, C.; Heldmann, M. Vasopressin modulates neural responses related to emotional stimuli in the right amygdala. Brain Res. 2013, 1499, 29–42. [Google Scholar] [CrossRef]
- Brunnlieb, C.; Nave, G.; Camerer, C.F.; Schosser, S.; Vogt, B.; Münte, T.F.; Heldmann, M. Vasopressin increases human risky cooperative behavior. Proc. Nat. Acad. Sci. USA 2016, 113, 2051–2056. [Google Scholar] [CrossRef] [Green Version]
- Rilling, J.K.; Demarco, A.C.; Hackett, P.D.; Chen, X.; Gautam, P.; Stair, S.; Haroon, E.; Thompson, R.; Ditzen, B.; Patel, R.; et al. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology 2014, 39, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Coccaro, E.F.; Kavoussi, R.J.; Hauger, R.L.; Cooper, T.B.; Ferris, C.F. Cerebrospinal fluid vasopressin levels: Correlates with aggression and serotonin function in personality-disordered subjects. Arch. Gen. Psychiatry 1998, 55, 708–714. [Google Scholar] [CrossRef] [Green Version]
- Dalton, K.M.; Nacewicz, B.M.; Johnstone, T.; Schaefer, H.S.; Gernsbacher, M.A.; Goldsmith, H.H.; Alexander, A.L.; Davidson, R.J. Gaze fixation and the neural circuitry of face processing in autism. Nat. Neurosci. 2005, 8, 519–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalton, K.M.; Nacewicz, B.M.; Alexander, A.L.; Davidson, R.J. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol. Psychiatry 2007, 61, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Hadjikhani, N.; Joseph, R.M.; Snyder, J.; Tager-Flusberg, H. Abnormal activation of the social brain during face perception in autism. Hum. Brain Mapp. 2007, 28, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Bookheimer, S.Y.; Wang, A.T.; Scott, A.; Sigman, M.; Dapretto, M. Frontal contributions to face processing differences in autism: Evidence from fMRI of inverted face processing. J. Int. Neuropsychol. Soc. 2008, 14, 922–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer-Lindenberg, A.; Kolachana, B.; Gold, B.; Olsh, A.; Nicodemus, K.K.; Mattay, V.; Dean, M.; Weinberger, D.R. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Mol. Psychiatry 2009, 14, 968–975. [Google Scholar] [CrossRef]
- Zink, C.F.; Stein, J.L.; Kempf, L.; Hakimi, S.; Meyer-Lindenberg, A. Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. J. Neurosci. 2010, 30, 7017–7022. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Carter, C.S.; Ying, J.; Bellugi, U.; Pournajafi-Nazarloo, H.; Korenberg, J.R. Oxytocin and vasopressin are dysregulated in Williams Syndrome, a genetic disorder affecting social behavior. PLoS ONE 2012, 7, e38513. [Google Scholar] [CrossRef] [Green Version]
- Rubin, L.H.; Carter, C.S.; Bishop, J.R.; Pournajafi-Nazarloo, H.; Harris, M.S.; Hill, S.K.; Reilly, J.L.; Sweeney, J.A. Peripheral vasopressin but not oxytocin relates to severity of acute psychosis in women with acutely-ill untreated first-episode psychosis. Schizophr. Res. 2013, 146, 138–143. [Google Scholar] [CrossRef] [Green Version]
- El-Ansfary, A.; Al-Ayadhi, L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J. Neuroinflamm. 2014, 11, 189. [Google Scholar] [CrossRef]
- Guastella, A.J.; Hickie, I.B. Oxytocin treatment, circuitry, and autism: A critical review of the literature placing oxytocin into the autism context. Biol. Psychiatry 2016, 79, 234–242. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, H.F.; Han, J.S.; Han, S.P. Genes related to oxytocin and arginine-vasopressin pathways: Associations with autism spectrum disorders. Neurosci. Bull. 2017, 33, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cataldo, I.; Azhari, A.; Esposito, G. A review of oxytocin and arginine-vasopressin receptors and their modulation of autism spectrum disorder. Front. Mol. Neurosci. 2018, 11, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, L.H.; Carter, C.S.; Bishop, J.R.; Pournajafi-Nazarloo, H.; Drogos, L.L.; Hill, S.K.; Reilly, J.L.; Sweeney, J.A. Reduced levels of vasopressin and reduced behavioral modulation of oxytocin in psychotic disorders. Schizophr. Bull. 2014, 40, 1374–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selten, J.P.; Cantor-Graae, E. Social defeat: Risk factor for schizophrenia? Br. J. Psychiatry 2005, 187, 101–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albers, H.E.; Karom, M.; Smith, D. Serotonin and vasopressin interact in the hypothalamus to control communicative behavior. Neuroreport 2002, 13, 931–933. [Google Scholar] [CrossRef]
- Galfi, M.; Radacs, M.; Juhasz, A.; Laszlo, F.; Molnar, A.; Laszlo, F.A. Serotonin-induced enhancement of vasopressin and oxytocin secretion in rat neurohypophyseal tissue culture. Regul. Pept. 2005, 127, 225–231. [Google Scholar] [CrossRef]
- Bachner-Melman, R.; Dina, C.; Zohar, A.H.; Constantini, N.; Lerer, E.; Hoch, S.; Sella, S.; Nemanov, L.; Gritsenko, I.; Lichtenberg, P.; et al. AVPR1a and SLC6A4 gene polymorphisms are associated with creative dance performance. PLoS Genet. 2005, 1, e42. [Google Scholar] [CrossRef] [Green Version]
- Pezawas, L.; Meyer-Lindenberg, A.; Drabant, E.M.; Verchinski, B.A.; Munoz, K.E.; Kolachana, B.S.; Egan, M.F.; Mattay, V.S.; Hariri, A.R.; Weinberger, D.R. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nat. Neurosci. 2005, 8, 828–834. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Buckholtz, J.W.; Kolachana, B.; Hariri, A.R.; Pezawas, L.; Blasi, G.; Wabnitz, A.; Honea, R.; Verchinski, B.; Callicott, J.H.; et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 6269–6274. [Google Scholar] [CrossRef] [Green Version]
- Grace, S.A.; Rossell, S.L.; Heinrichs, M.; Kordsachia, C.; Labuschagne, I. Oxytocin and brain activity in humans: A systematic review and coordinate based meta-analysis of functional MRI studies. Psychoneuroendocrinology 2018, 96, 6–24. [Google Scholar] [CrossRef]
- Lefevre, A.; Hurlemann, R.; Grinevich, V. Imaging neuropeptide effects on human brain function. Cell Tissue Res. 2018, 375, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Brydges, N.M.; Hall, J.; Best, C.; Rule, L.; Watkin, H.; Drake, A.J.; Lewis, C.; Thomas, K.L.; Hall, J. Childhood stress impairs social function through AVP-dependent mechanisms. Transl. Psychiatry 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Meinlschmidt, G.; Heim, C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biol. Psychiatry 2007, 61, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R.; Young, L.J. The neurobiology of attachment. Nat. Rev. Neurosci. 2001, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Goossens, L.; Kukolja, J.; Onur, O.A.; Fink, G.R.; Maier, W.; Griez, E.; Schruers, K.; Hurlemann, R. Selective processing of social stimuli in the superficial amygdala. Hum. Brain Mapp. 2009, 30, 3332–3338. [Google Scholar] [CrossRef]
- Rogers, C.N.; Ross, A.P.; Sahu, S.P.; Siegel, E.R.; Dooyema, J.M.; Cree, M.A.; Stopa, E.G.; Young, L.J.; Rilling, J.K.; Albers, H.E.; et al. Oxytocin-and arginine vasopressin-containing fibers in the cortex of humans, chimpanzees, and rhesus macaques. Am. J. Primatol. 2018, 80, e22875. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and function of the human insula. J. Clin. Neurophysiol. 2017, 34, 300–306. [Google Scholar] [CrossRef]
- Thibonnier, M.; Graves, M.K.; Wagner, M.S.; Chatelain, N.; Soubrier, F.; Corvol, P.; Willard, H.F.; Jeunemaitre, X. Study of V1-vascular vasopressin receptor gene microsatellite polymorphisms in human essential hypertension. J. Mol. Cell. Cardiol. 2000, 32, 557–564. [Google Scholar] [CrossRef]
- Kim, S.J.; Young, L.J.; Gonen, D.; Veenstra-VanderWeele, J.; Courchesne, R.; Lord, C.; Leventhal, B.L.; Cook, E.H., Jr.; Insel, T.R. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol. Psychiatry 2002, 7, 503–507. [Google Scholar] [CrossRef] [Green Version]
- Wassink, T.H.; Piven, J.; Vieland, V.J.; Pietila, J.; Goedken, R.J.; Folstein, S.E.; Sheffield, V.C. Examination of AVPR1a as an autism susceptibility gene. Mol. Psychiatry 2004, 9, 968–972. [Google Scholar] [CrossRef]
- Hammock, E.A.; Young, L.J. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science 2005, 308, 1630–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabry, K.E.; Streatfeild, C.A.; Keane, B.; Solomon, N.G. Avpr1a length polymorphism is not associated with either social or genetic monogamy in free-living prairie voles. Anim. Behav. 2011, 81, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walum, H.; Westberg, L.; Henningson, S.; Neiderhiser, J.M.; Reiss, D.; Igl, W.; Ganiban, J.M.; Spotts, E.L.; Pedersen, N.L.; Eriksson, E.; et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc. Natl Acad. Sci. USA 2008, 105, 14153–14156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guastella, A.J.; Kenyon, A.R.; Unkelbach, C.; Alvares, G.A.; Hickie, I.B. Arginine vasopressin selectively enhances recognition of sexual cues in male humans. Psychoneuroendocrinology 2011, 36, 294–297. [Google Scholar] [CrossRef]
- Zink, C.F.; Meyer-Lindenberg, A. Human neuroimaging of oxytocin and vasopressin in social cognition. Horm. Behav. 2012, 61, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Ben-Efraim, Y.J.; Wasserman, D.; Wasserman, J.; Sokolowski, M. Family-based study of AVPR1B association and interaction with stressful life events on depression and anxiety in suicide attempts. Neuropsychopharmacology 2013, 38, 1504–1511. [Google Scholar] [CrossRef] [Green Version]
- Aspé-Sánchez, M.; Moreno, M.; Rivera, M.I.; Rossi, A.; Ewer, J. Oxytocin and vasopressin receptor gene polymorphisms: Role in social and psychiatric traits. Front. Neurosci. 2016, 9, 510. [Google Scholar] [CrossRef] [Green Version]
- Jobst, A.; Dehning, S.; Ruf, S.; Notz, T.; Buchheim, A.; Henning-Fast, K.; Meißner, D.; Meyer, S.; Bondy, B.; Müller, N.; et al. Oxytocin and vasopressin levels are decreased in the plasma of male schizophrenia patients. Acta Neuropsychiatr. 2014, 26, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Born, J.; Lange, T.; Kern, W.; McGregor, G.P.; Bickel, U.; Fehm, H.L. Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci. 2002, 5, 514–516. [Google Scholar] [CrossRef]
- Glover, G.H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.; Gupta, S.; Miller, K.; Mills, S.; Orr, S. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology 2004, 29, 35–48. [Google Scholar] [CrossRef]
- Thompson, R.R.; George, K.; Walton, J.C.; Orr, S.P.; Benson, J. Sex-specific influences of vasopressin on human social communication. Proc. Natl. Acad. Sci. USA 2006, 103, 7889–7894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rilling, J.K.; DeMarco, A.C.; Hackett, P.D.; Thompson, R.; Ditzen, B.; Patel, R.; Pagnoni, G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology 2012, 37, 447–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Anders, S.M.; Goodson, J.L.; Kingsbury, M.A. Beyond “oxytocin= good”: Neural complexities and the flipside of social bonds. Arch. Sex. Behav. 2013, 42, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; DeMarco, A.C.; Haroon, E.; Rilling, J.K. Neuroticism modulates the effects of intranasal vasopressin treatment on the neural response to positive and negative social interactions. Neuropsychologia 2015, 73, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Sheng, Y.; Feng, X.; Wu, J. The effects and safety of vasopressin receptor agonists in patients with septic shock: A meta-analysis and trial sequential analysis. Crit. Care. 2019, 23, 91. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.Y.; Wo, N.C.; Stoev, S.T.; Cheng, L.L.; Manning, M. Discovery and design of novel and selective vasopressin and oxytocin agonists and antagonists: The role of bioassays. Exp. Physiol. 2000, 85, 7S–18S. [Google Scholar] [CrossRef]
- Rondon-Berrios, H.; Berl, T. Vasopressin receptor antagonists: Characteristics and clinical role. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 289–303. [Google Scholar] [CrossRef]
- Narayen, G.; Mandal, S.N. Vasopressin receptor antagonists and their role in clinical medicine. Indian J. Endocrinol. Metab. 2012, 16, 183–191. [Google Scholar] [CrossRef]
- Wu, M.Y.; Chen, T.T.; Chen, Y.C.; Tarng, D.C.; Wu, Y.C.; Lin, H.H.; Tu, Y.K. Effects and safety of oral tolvaptan in patients with congestive heart failure: A systematic review and network meta-analysis. PLoS ONE 2017, 12, e0184380. [Google Scholar] [CrossRef] [Green Version]
- Farah, J.; Daifallah, S.; Zmily, H.; Ghali, J.K. An overview of satavaptan: A selective V2 receptor antagonist. Therapy 2010, 7, 409–422. [Google Scholar] [CrossRef]
- Rangarajan, B.; Binoy, V.; Hingmire, S.S.; Noronha, V. Tolvaptan. South Asian J. Cancer 2014, 3, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Di Mise, A.; Venneri, M.; Ranieri, M.; Centrone, M.; Pellegrini, L.; Tamma, G.; Valenti, G. Lixivaptan, a new generation diuretic, counteracts vasopressin-induced aquaporin-2 trafficking and function in renal collecting duct cells. Int. J. Mol. Sci. 2019, 21, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, F.; Raufi, A.M.; Washington, B.; Ghali, J.K. Conivaptan: A dual receptor vasopressin V1a/V2 antagonist. Cardiovasc. Drug Rev. 2007, 25, 261–279. [Google Scholar]
- Finley, J.J.; Konstam, M.A.; Udelson, J.E. Arginine vasopressin antagonists for the treatment of heart failure and hyponatremia. Circulation 2008, 118, 410–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolognani, F.; Del valle, R.M.; Squassante, L.; Wandel, C.; Derks, M.; Murtagh, L. A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci. Transl. Med. 2019, 11, eaat7838. [Google Scholar] [CrossRef] [PubMed]
- Schnider, P.; Bissantz, C.; Bruns, A.; Dolente, C.; Goetschi, E.; Jakob-Roetne, R.; Kunnecke, B.; Mueggler, T.; Muster, W.; Parrott, N.; et al. Discovery of balovaptan, a vasopressin 1a receptor antagonist for the treatment of autism spectrum disorder. J. Med. Chem. 2020, 63, 1511–1525. [Google Scholar] [CrossRef]
- Steinwall, M.; Bossmar, T.; Brouard, R.; Laudanski, T.; Olofsson, P.; Urban, R.; Wolff, K.; Le-Fur, G.; Akerlund, M. The effect of relcovaptan (SR 49059), an orally active vasopressin V1a receptor antagonist, on uterine contractions in preterm labor. Gynecol. Endocrinol. 2005, 20, 104–109. [Google Scholar] [CrossRef]
- Decaux, G.; Soupart, A.; Vassart, G. Non-peptide arginine-vasopressin antagonists: The vaptans. Lancet. 2008, 371, 1624–1632. [Google Scholar] [CrossRef]
- Serradeil-Le Gal, C.; Wagnon, J.; Tonnerre, B.; Roux, R.; Garcia, G.; Griebel, G.; Aulombard, A. An overview of SSR149415, a selective nonpeptide vasopressin V(1b) receptor antagonist for the treatment of stress-related disorders. CNS Drug Rev. 2005, 11, 53–68. [Google Scholar]
- Schule, C.; Baghai, T.C.; Eser, D.; Rupprecht, R. Hypothalamic–pituitary–adrenocortical system dysregulation and new treatment strategies in depression. Exp. Rev. Neurother. 2009, 9, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Rihakova, L.; Quiniou, C.; Hamdan, F.F.; Kaul, R.; Brault, S.; Hou, X.; Lahaie, I.; Sapieha, P.; Hamel, D.; Shao, Z.; et al. VRQ397 (CRAVKY): A novel noncompetitive V2 receptor antagonist. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1009–R1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamura, Y.; Nakamura, S.; Itoh, S.; Hirano, T.; Onogawa, T.; Yamashita, T.; Yamada, Y.; Tsujimae, M.; Aoyama, M.; Kotosai, K.; et al. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: Pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J. Pharmacol. Exp. Ther. 1998, 287, 860–867. [Google Scholar] [PubMed]
- Miyazaki, T.; Fujiki, H.; Yamamura, Y.; Nakamura, S.; Mori, T. Tolvaptan, an orally active vasopressin V2-receptor antagonist—pharmacology and clinical trials. Cardiovasc. Drug Rev. 2007, 25, 1–13. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef] [Green Version]
- Pharos. Available online: https://pharos.nih.gov/ (accessed on 16 October 2020).
- Albright, J.D.; Reich, M.F.; Delos Santos, E.G.; Dusza, J.P.; Sum, F.W.; Venkatesan, A.M.; Coupet, J.; Chan, P.S.; Ru, X.; Mazandarani, H.; et al. 5-Fluoro-2-methyl-N-[4-(5H-pyrrolo[2,1-c]-[1,4]benzodiazepin-10(11H)-ylcarbonyl)-3-chlorophenyl]benzamide (VPA-985): An orally active arginine vasopressin antagonist with selectivity for V2 receptors. J. Med. Chem. 1998, 41, 2442–2444. [Google Scholar] [CrossRef]
- Ku, E.; Nobakht, N.; Campese, V.M. Lixivaptan: A novel vasopressin receptor antagonist. Drug Eval. 2009, 18, 657–662. [Google Scholar] [CrossRef]
- Serradeil-Le Gal, C.; Lacour, C.; Valette, G.; Garcia, G.; Foulon, L.; Galindo, G.; Bankir, L.; Pouzet, B.; Guillon, G.; Barberis, C.; et al. Characterization of SR121463A, a highly potent and selective, orally active vasopressin V2 receptor antagonist. J. Clin. Investig. 1996, 98, 2729–2738. [Google Scholar] [CrossRef]
- Bernat, A.; Hoffman, P.; Dumas, A.; Serradeil-le Gal, C.; Raufaste, D.; Herbert, J.M. V2 receptor antagonism of DDAVP-induced release of hemostasis factors in conscious dogs. J. Pharmacol. Exp. Ther. 1997, 282, 597–602. [Google Scholar]
- Serradeil-Le Gal, C.; Raufaste, D.; Double-Cazanave, E.; Guillon, G.; Garcia, C.; Pascal, M.; Maffrand, J.P. Binding properties of a selective tritiated vasopressin V2 receptor antagonist, [3H]-SR121463. Kidney Int. 2000, 56, 1613–1622. [Google Scholar] [CrossRef] [Green Version]
- Serradeil-Le Gal, C. An overview of SR121463, a selective non-peptide vasopressin V(2) receptor antagonist. Cardiovasc. Drug Rev. 2001, 19, 201–214. [Google Scholar] [PubMed]
- Gines, P.; Wong, F.; Watson, H.; Milutinovic, S.; Ruiz del Arbol, L.; Olteanu, D. Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: A randomized trial. Hepatology 2008, 48, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Yatsu, T.; Tomura, Y.; Tahara, A.; Wada, K.; Kusayama, T.; Tsukada, J.; Tokioka, T.; Uchida, W.; Inagaki, O.; Iizumi, Y.; et al. Cardiovascular and renal effects of conivaptan hydrochloride (YM087), a vasopressin V1A and V2 receptor antagonist, in dogs with pacing-induced congestive heart failure. Eur. J. Pharmacol. 1999, 376, 239–246. [Google Scholar] [CrossRef]
- Wada, K.; Fujimori, A.; Matsukawa, U.; Arai, Y.; Sudoh, K.; Yatsu, T.; Sasamata, M.; Miyata, K. Intravenous administration of conivaptan hydrochloride improves cardiac hemodynamics in rats with myocardial infarction-induced congestive heart failure. Eur. J. Pharmacol. 2005, 507, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Crombie, A.L.; Antrilli, T.M.; Campbell, B.A.; Crandall, D.L.; Failli, A.A.; He, Y.; Kern, J.C.; Moore, W.J.; Nogle, L.M.; Trybulski, E.J. Synthesis and evaluation of azabicyclo[3.2.1]octane derivatives as potent mixed vasopressin antagonists. Bioorg. Med. Chem. Lett. 2010, 20, 3742–3745. [Google Scholar] [CrossRef]
- Serradeil-Le Gal, C.; Wagnon, J.; Simiand, J.; Griebel, G.; Lacour, C.; Guillon, G.; Barberis, C.; Brossard, G.; Soubrie, P.; Nisato, D.; et al. Characterization of (2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl)-2-oxo-2,3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide (SSR149415), a selective and orally active vasopressin V1b receptor antagonist. J. Pharmacol. Exp. Ther. 2002, 300, 1122–1130. [Google Scholar]
- Ratni, H.; Rogers-Evans, M.; Bissantz, C.; Grundschober, C.; Moreau, J.L.; Schuler, F.; Fischer, H.; Alvarez Sanchez, R.; Schnider, P. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J. Med. Chem. 2015, 58, 2275–2289. [Google Scholar] [CrossRef]
- Akerlund, M.; Bossmar, T.; Brouard, R.; Kostrzewska, A.; Laudanski, T.; Lemancewicz, A.; Serradeil-Le Gal, C.; Steinwall, M. Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects on isolated myometrium from preterm and term pregnant women. Br. J. Obstet. Gynaecol. 1999, 106, 1047–1053. [Google Scholar] [CrossRef]
- Cotte, N.; Balestre, M.N.; Aumelas, A.; Mahé, E.; Phalipou, S.; Morin, D.; Hibert, M.; Manning, M.; Durroux, T.; Barberis, C.; et al. Conserved aromatic residues in the transmembrane region VI of the V1a vasopressin receptor differentiate agonist vs. antagonist ligand binding. Eur. J. Biochem. 2000, 267, 4253–4263. [Google Scholar] [CrossRef]
- Griffante, C.; Green, A.; Curcuruto, O.; Haslam, C.P.; Dickinson, B.A.; Arban, R. Selectivity of d[Cha4]AVP and SSR149415 at human vasopressin and oxytocin receptors: Evidence that SSR149415 is a mixed vasopressin V1b/oxytocin receptor antagonist. Br. J. Pharmacol. 2005, 146, 744–751. [Google Scholar] [CrossRef]
- Yamamura, Y.; Ogawa, H.; Yamashita, H.; Chihara, T.; Miyamoto, H.; Nakamura, S.; Onogawa, T.; Yamashita, T.; Hosokawa, T.; Mori, T.; et al. Characterization of a novel aquaretic agent, OPC-31260, as an orally effective, nonpeptide vasopressin V2 receptor antagonist. Br. J. Pharmacol. 1992, 105, 787–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotte, N.; Balestre, M.N.; Phalipou, S.; Hibert, M.; Manning, M.; Barberis, C.; Mouillac, B. Identification of residues responsible for the selective binding of peptide antagonists and agonists in the V2 vasopressin receptor. J. Biol. Chem. 1998, 273, 29462–29468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, K.; Shijubo, N.; Kodama, T.; Mori, K.; Sugiura, T.; Kuriyama, T.; Kawahara, M.; Shinkai, T.; Iguchi, H.; Sakurai, M. Clinical implication of the antidiuretic hormone (ADH) receptor antagonist mozavaptan hydrochloride in patients with ectopic ADH syndrome. Jpn. J. Clin. Oncol. 2011, 41, 148–152. [Google Scholar] [PubMed]
- Rai, A.; Whaley-Connell, A.; McFarlane, S.; Sowers, J.R. Hyponatremia, arginine vasopressin dysregulation, and vasopressin receptor antagonism. Am. J. Nephrol. 2006, 26, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Wang, X.; Qian, Q.; Somlo, S.; Harris, P.C.; Gattone, V.H., II. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat. Med. 2004, 10, 363–364. [Google Scholar] [CrossRef]
- Meijer, E.; Gansevoort, R.T.; De Jong, P.E.; van der Wal, A.M.; Leonhard, W.N.; de Krey, S.R.; van den Born, J.; Mulder, G.M.; van Goor, H.; Struck, J.; et al. Therapeutic potential of vasopressin V2 receptor antagonist in a mouse model for autosomal dominant polycystic kidney disease: Optimal timing and dosing of the drug. Nephrol. Dial. Transplant. 2011, 26, 2445–2453. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gattone II, V.; Harris, P.C.; Torres, V.E. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J. Am. Soc. Nephrol. 2005, 16, 846–851. [Google Scholar] [CrossRef] [Green Version]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Dandurand, A.; Ouyang, J.; Czerwiec, F.S.; Blais, J.D. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: The TEMPO 4:4 Trial. Nephrol. Dial. Transplant 2018, 33, 477–489. [Google Scholar] [CrossRef] [Green Version]
- Watkins, P.B.; Lewis, J.H.; Kaplowitz, N.; Alpers, D.H.; Blais, J.D.; Smotzer, D.M.; Krasa, H.; Ouyang, J.; Torres, V.E.; Czerwiec, F.S.; et al. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: Analysis of clinical trials database. Drug Saf. 2015, 38, 1103–1113. [Google Scholar] [CrossRef] [Green Version]
- Sans-Atxer, L.; Joly, D. Tolvaptan in the treatment of autosomal dominant polycystic kidney disease: Patient selection and special considerations. Int. J. Nephrol. Renovasc. Dis. 2018, 11, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Y.; Wang, Z.M.; Huang, Y. Polycystic liver disease: Classification, diagnosis, treatment process, and clinical management. World J. Hepatol. 2020, 12, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Manu, P.; Olshanskiy, V.; Napolitano, B.; Kane, J.M.; Malhotra, A.K. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 2009, 302, 1765–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, K.J.; Oztan, O.; Libove, R.A.; Mohsin, N.; Karhson, D.S.; Sumiyoshi, R.D.; Summers, J.E.; Hinman, K.E.; Motonaga, K.S.; Phillips, J.M.; et al. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci. Transl. Med. 2019, 11, eaau7356. [Google Scholar] [CrossRef] [PubMed]
- Noskov, V.B.; Larina, I.M.; Nichiporuk, I.A.; Pastushkova, L.K.; Vasilieva, G. Evaluation of synthetic antidiuretic hormone as a corrective substance following a head-down tilt. Hum. Physiol. 2012, 38, 781–785. [Google Scholar] [CrossRef]
- Kamperis, K.; Van Herzeele, C.; Rittig, S.; Walle, J.V. Optimizing response to desmopressin in patients with monosymptomatic nocturnal enuresis. Pediatric. Nephrol. 2017, 32, 217–226. [Google Scholar]
- Leone, M.; Albanese, J.; Delmas, A.; Chaabane, W.; Garnier, F.; Martin, C. Terlipressin in catecholamine resistant septic shock patients. Shock 2004, 22, 314–319. [Google Scholar] [CrossRef]
- Sarin, S.K.; Sharma, P. Terlipressin: An asset for hepatologists! Hepatology 2011, 54, 724–728. [Google Scholar] [CrossRef]
- Scarpati, G.; Piazza, O. Vasopressin vs terlipressin in treatment of refractory shock. Transl. Med. UniSa. 2013, 5, 22–27. [Google Scholar]
- Jamil, K.; Pappas, S.C.; Devarakonda, K.R. In vitro binding and receptor-mediated activity of terlipressin at vasopressin receptors V1 and V2. J. Exp. Pharmacol. 2018, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Olgart, L.; Gazelius, B. Effects of adrenaline and felypressin (octapressin) on blood flow and sensory nerve activity in the tooth. Acta Odontol. Scand. 1977, 35, 69–75. [Google Scholar] [CrossRef]
- Cecanho, R.; De Luca Jr, L.A.; Ranali, J. Cardiovascular effects of felypressin. Anesth. Prog. 2006, 53, 119–125. [Google Scholar] [CrossRef]
- Bronzo, A.L.A.; Cardoso, C.G.; Ortega, K.C.; Mion, D. Felypressin increases blood pressure during dental procedures in hypertensive patients. Arq. Bras. Cardiol. 2012, 99, 724–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, M.; Misicka, A.; Olma, A.; Bankowski, K.; Stoev, S.; Chini, B.; Durroux, T.; Mouillac, B.; Corbani, M.; Guillon, G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol. 2012, 24, 609–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Target Tissue | Receptor | Function | References |
|---|---|---|---|
| Kidney—Macula densa, intermediate, distal and collector tubules | V2R | Signal transduction, AQP2 shuttling to cell surface and water permeability, AQP2 mRNA synthesis, intracellular cAMP regulation | [14,53,54,55,56] |
| Kidney—Mesangial cells, efferent arterioles, renal tubules | V1aR | Vasoconstriction | [57,58,59] |
| Kidney | V1bR | Unknown | [16,60,61] |
| Vascular Smooth Muscle | V1aR | Vasoconstriction, myocardial hypertrophy, V1aR mRNA upregulation, hypertension | [53,54,58,62] |
| V2R | Vasodilation | [53] | |
| Brain—Anterior Pituitary | V1bR | ACTH secretion, stimulation of endocrine response to stress | [17,53,63,64,65] |
| Brain—diffused expression | V1aR | Regulation of emotional and adaptive behaviors, pain | [66,67,68,69] |
| Brain—HPA axis (adrenal cortex) | V1aR | Cortisol synthesis and secretion | [70] |
| Brain—SCN | V1aR | Circadian rhythm | [71] |
| Brain—Cerebellum (rats) | V2R | Unknown | [55] |
| Pancreas | V1bR | Glucagon release, intracellular Ca2+ regulation, cell proliferation | [54,72] |
| Liver—Hepatocytes | V1aR | Glycogenolysis | [18,53] |
| Blood—Platelets | V1aR | Platelet aggregation | [53,73] |
| Blood—Leukocytes | V1aR | Chemotaxis, chemokine and antibody production | [74,75] |
| Myometrium | V1aR | Uterine contraction | [53,76] |
| Prostate | V1aR | Unknown, causative upregulation in castration resistant prostate cancer | [18,77] |
| Skeletal muscle | V1aR | Unknown | [18] |
| Lung | V1aR V2R | Unknown Anti-inflammatory | [58] [78] |
| Bone | V2R | Bone remodeling | [79] |
| Cervical ganglion (rat) | V1aR | Unknown | [80,81] |
| Spleen (rat) | V1aR | Unknown | [81] |
| Gonads (rat) | V1aR | Unknown | [80,82] |
| AVP Receptor Antagonists | Chemistry | Binding Affinity & MoA | Route of Administration, Dosage & Physiology | Reference(s) |
|---|---|---|---|---|
Tolvaptan | Empirical formula: C26H25ClN3O2Chemical nomenclature: N[4 -(7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1-benzazepine-1-carbonyl)-3-methylphenyl]-2-methylbenzamide | Binds V2R (pKi: 8.9–9.4) with 30-fold higher affinity than V1aR in vivo. Reduces water reabsorption (renal collecting ducts), promotes aquaresis. | Oral 15–60 mg/day Reduced rate of renal function decline, edema, body weight and serum Na2+ levels. | [130,306,308,311,319,320,321,322] |
Lixivaptan | Empirical formula: C27H21ClFN3O2 Chemical nomenclature: N-[3-chloro-4-(6,11-dihydropyrrolo[2,1-c][1,4]benzodiazepine-5-carbonyl)phenyl]-5-fluoro-2-methylbenzamide | Binds V2R (pKi: 8.9–9.2) with 100-fold higher affinity than V1aR in vivo. Prevents translocation and localization of AQP2 to renal collecting ducts, promotes aquaresis. | Oral 30–150 mg/day or 1–10 mg/kg | [309,311,323,324] |
Satavaptan | Empirical formula: C32H26N4O2 Chemical nomenclature: N-tert-butyl-4-[5′-ethoxy-4-(2-morpholin-4ylethoxy)-2′oxospiro [cyclohexane-1,3′-indole]-1′-yl]sulfonyl-3-methoxybenzamide | Binds V2R (pKi: 8.4–9.3) with 112-fold greater affinity than V1aR in vivo. Aquaretic, similar to tolvaptan. | Oral 5–25 mg/day | [307,311,325,326,327,328,329] |
Conivaptan | Empirical formula: C33H45N3O8S Chemical nomenclature: N-[4-(2-methyl-4,5-dihydro-3H-imidazo [4,5-d][1]benzazepine-6-carbonyl)phenyl]-2-phenylbenzamide | High affinity for both V1aR (pKi: 9.37) and V2R (pKi: 9.44) in vivo. V1aR: reduces Ca2+ mobilization and kinase activity (cardiac tissue), reduces myocardium hypertrophy. V2R: promotes aquaresis similar to tolvaptan. | Oral & intravenous 20–40 mg/day or 0.003–0.1 mg/kg | [310,311,322,330,331,332] |
Nelivaptan | Empirical formula: C30H32ClN3O8S Chemical nomenclature: (2S,4R)-1-[(3R)-5-chloro-1-(2,4-dimethoxyphenyl) sulfonyl-3-(2-methoxyphenyl)-2-oxoindol-3-yl]-4-hydroxy-N,N-dimethylpyrrolidine-2-carboxamide | Binding affinity for V1bR (pKi: 5.9) in vivo. Normalizes ACTH hypersecretion in response to stress stimuli. | Oral 3–30 mg/day Potential anxiolytic and anti-depressant. | [64,316,317,333] |
Balovaptan | Empirical formula: C22H24ClN5O Chemical nomenclature: 8-chloro-5-methyl-1-(4-pyridin-2-yloxycyclohexyl)-4,6-dihydro-[1,2,4] triazolo[4,3a][1,4]benzodiazepine | Binding affinity for V1aR (pKd: 5.0, Ki: 1nM) in vivo. | Oral 10 mg/day Improved Vineland II adaptive behavior scales. | [312,313,334] |
Relcovaptan | Empirical formula: C28H27Cl2N5O7S Chemical nomenclature: (2S)-1-[(2R,3S)-5-chloro -3-(2-chlorophenyl)-1-(3,4-dimethoxyphenyl) sulfonyl-3-hydroxy-2H-indole-2-carbonyl] pyrrolidine-2-carboxamide | Binding affinity for V1aR (pKi: 8.1) in vivo. Promotes vasodilation (vascular bed). | Oral 400 mg/day Potential for treatment of Raynaud’s syndrome and regulation of uterine contractions in preterm labor. | [314,315,335,336,337] |
Mozavaptan | Empirical formula: C27H29N3O2 Chemical nomenclature: N-[4-[5-(dimethylamino)-2,3,4,5-tetrahydro-1-benzazepine-1-carbonyl]phenyl]-2-methylbenzamide | Binding affinity for V2R (pKi: 8.03) in vivo. Aquaretic. | Oral 30 mg/day or 1–30 mg/kg | [315,322,338,339,340] |
| AVP & Synthetic Analogs | Chemistry | Binding Affinity & MoA | Route of Administration, Dosage & Physiology | Reference(s) |
|---|---|---|---|---|
AVP  | Empirical formula: C44H61N13O12S2 Chemical nomenclature: (2S)-1-[(4R,7S,10S,13S,16S,19R)-19-amino-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-benzyl-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]-N-[(2S)-1-[(2-amino-2-oxoethyl)amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]pyrrolidine-2-carboxamide | Binds V1aR (pKi: 9.59), V1bR (pKi: 9.31) and V2R (pKi: 8.92) with high affinity. MoA: See text for details. | Oral, intravenous & intranasal 24–32 IU/day | [322,350] |
Desmopressin  | Empirical formula: C46H64N14O12S2 Chemical nomenclature: (2S)-N-[(2R)-1-[(2-amino-2-oxoethyl)amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]-1-[(4R,7S,10S,13S,16S)-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-benzyl-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]pyrrolidine-2-carboxamide | Binding affinity for V2R (pKi: 8.3). Induces AQP2 apical translo-cation (renal collecting duct) and water reabsorption. | Oral & intranasal 0.2–0.6 mg/day Need frequent medical follow-up. | [322,339,351,352] |
Terlipressin  | Empirical formula: C52H74N16O15S2 Chemical nomenclature: (2S)-1-[(4R,7S,10S,13S,16S,19R)-19-[[2-[[2-[(2-aminoacetyl)amino]acetyl]amino]acetyl]amino]-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-benzyl-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]-N-[(2S)-6-amino-1-[(2-amino-2-oxoethyl)amino]-1-oxohexan-2-yl]pyrrolidine-2-carboxamide | Binding affinity for both V1aR (1.1 × 10−6 Ki moles) and V2R (6.9 × 10−6 Ki moles). V1aR: induces splanchnic and renal vasocon-striction, reduces portal pressure. V2R: increases apical AQP2 and water retention. | Intravenous 1–2 mg/day as needed. Increased mean arterial pressure, decreased heart rate and improved renal function. | [353,354,355,356] |
Felypressin  | Empirical formula: C46H65N13O11S2 Chemical nomenclature: (2S)-N-[(2S)-6-amino-1-[(2-amino-2-oxoethyl)amino]-1-oxohexan-2-yl]-1-[(4R,7S,10S,13S,16S,19R)-19-amino-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13,16-dibenzyl-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]pyrrolidine-2-carboxamide | Binding affinity for V1aR (21 units/mg). Induces smooth muscle contraction (vascular bed). | Intramuscular 240 ng/kg Less antidiuretic effects than AVP. | [357,358,359,360] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparapani, S.; Millet-Boureima, C.; Oliver, J.; Mu, K.; Hadavi, P.; Kalostian, T.; Ali, N.; Avelar, C.M.; Bardies, M.; Barrow, B.; et al. The Biology of Vasopressin. Biomedicines 2021, 9, 89. https://doi.org/10.3390/biomedicines9010089
Sparapani S, Millet-Boureima C, Oliver J, Mu K, Hadavi P, Kalostian T, Ali N, Avelar CM, Bardies M, Barrow B, et al. The Biology of Vasopressin. Biomedicines. 2021; 9(1):89. https://doi.org/10.3390/biomedicines9010089
Chicago/Turabian StyleSparapani, Samantha, Cassandra Millet-Boureima, Joshua Oliver, Kathy Mu, Pegah Hadavi, Tamar Kalostian, Nazifa Ali, Carla Maria Avelar, Marion Bardies, Brenton Barrow, and et al. 2021. "The Biology of Vasopressin" Biomedicines 9, no. 1: 89. https://doi.org/10.3390/biomedicines9010089