Sex-Dependent Changes in Right Ventricular Gene Expression in Response to Pressure Overload in a Rat Model of Pulmonary Trunk Banding
Abstract
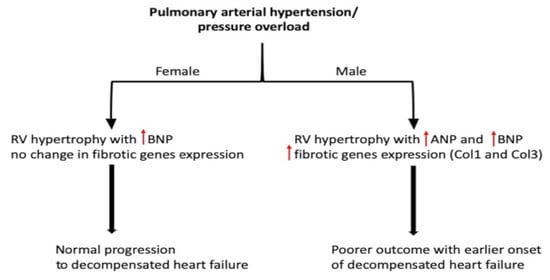
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Pulmonary Trunk Banding
2.4. Evaluation of Hemodynamic
2.5. Evaluation of Anatomic Measures
2.6. Measurement of Right Ventricular Hypertrophy
2.7. Gene Expression
2.8. Pulmonary Artery Remodeling
2.9. Statistical Analysis
3. Results
3.1. Effect of Sex and PTB on Physiological Parameters
3.2. Effect of Sex and PTB on Cardiac Function
3.3. Effect of PTB on RV Gene Expression
3.4. Effect of PTB on Lung Gene Expression
3.5. Effect of PTB on Lung’s Microvascular Remodeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANP | Natriuretic peptide A |
| BNP | Natriuretic peptide B |
| B2M | β-2-microglobulin |
| Col1 | Collagen 1 |
| Col3 | Collagen 3 |
| CO | Cardiac output |
| Ea | Arterial elastance |
| Eed | End-diastolic elastance |
| Ees | End-systolic elastance |
| ESP | End systolic pressure |
| F.I | Fulton index |
| FN | Fibronectin |
| HR | Heart Rate |
| LV | Left ventricle |
| PA | Pulmonary artery |
| PAH | Pulmonary arterial hypertension |
| PH | Pulmonary hypertension |
| PTB | Pulmonary trunk banding |
| RHF | Right heart failure |
| RV | Right ventricle |
| RVH | Right ventricular hypertrophy |
| RVSP | Right ventricular systolic pressure |
| SV | Stroke volume |
| TAPSE | Tricuspid annular plane systolic excursion |
| VTI | Velocity time integral |
References
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R.; Manes, A. The right ventricle in pulmonary arterial hypertension. Eur. Respir. Rev. 2014, 23, 476–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vonk Noordegraaf, A.; Galie, N. The role of the right ventricle in pulmonary arterial hypertension. Eur. Respir. Rev. 2011, 20, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGoon, M.D.; Miller, D.P. Reveal: A contemporary US pulmonary arterial hypertension registry. Eur. Respir. Rev. 2012, 21, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Subias, P.; Blanco, I.; Lopez-Meseguer, M.; Lopez-Guarch, C.J.; Roman, A.; Morales, P.; Castillo-Palma, M.J.; Segovia, J.; Gomez-Sanchez, M.A.; Barbera, J.A.; et al. Survival in pulmonary hypertension in Spain: Insights from the Spanish registry. Eur. Respir. J. 2012, 40, 596–603. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Huscher, D.; Ghofrani, H.A.; Delcroix, M.; Distler, O.; Schweiger, C.; Grunig, E.; Staehler, G.; Rosenkranz, S.; Halank, M.; et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: Results from the COMPERA registry. Int. J. Cardiol 2013, 168, 871–880. [Google Scholar] [CrossRef]
- Skride, A.; Sablinskis, K.; Lejnieks, A.; Rudzitis, A.; Lang, I. Characteristics and survival data from Latvian pulmonary hypertension registry: Comparison of prospective pulmonary hypertension registries in Europe. Pulm. Circ. 2018, 8, 2045894018780521. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, W.; van de Veerdonk, M.C.; Trip, P.; de Man, F.; Heymans, M.W.; Marcus, J.T.; Kawut, S.M.; Bogaard, H.J.; Boonstra, A.; Vonk Noordegraaf, A. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest 2014, 145, 1230–1236. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, S.; Traiger, G.L.; Turner, M.; McGoon, M.D.; Wason, P.; Barst, R.J. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest 2012, 141, 363–373. [Google Scholar] [CrossRef]
- Kjellstrom, B.; Nisell, M.; Kylhammar, D.; Bartfay, S.E.; Ivarsson, B.; Radegran, G.; Hjalmarsson, C. Sex-specific differences and survival in patients with idiopathic pulmonary arterial hypertension 2008–2016. ERJ Open Res. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Kawut, S.M.; Lima, J.A.; Barr, R.G.; Chahal, H.; Jain, A.; Tandri, H.; Praestgaard, A.; Bagiella, E.; Kizer, J.R.; Johnson, W.C.; et al. Sex and race differences in right ventricular structure and function: The multi-ethnic study of atherosclerosis-right ventricle study. Circulation 2011, 123, 2542–2551. [Google Scholar] [CrossRef] [Green Version]
- Ventetuolo, C.E.; Ouyang, P.; Bluemke, D.A.; Tandri, H.; Barr, R.G.; Bagiella, E.; Cappola, A.R.; Bristow, M.R.; Johnson, C.; Kronmal, R.A.; et al. Sex hormones are associated with right ventricular structure and function: The MESA-right ventricle study. Am. J. Respir. Crit. Care Med. 2011, 183, 659–667. [Google Scholar] [CrossRef]
- Hemnes, A.R.; Maynard, K.B.; Champion, H.C.; Gleaves, L.; Penner, N.; West, J.; Newman, J.H. Testosterone negatively regulates right ventricular load stress responses in mice. Pulm. Circ. 2012, 2, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Hester, J.; Ventetuolo, C.; Lahm, T. Sex, Gender, and Sex Hormones in Pulmonary Hypertension and Right Ventricular Failure. Compr. Physiol. 2019, 10, 125–170. [Google Scholar] [PubMed]
- Andersen, A.; Nielsen, J.M.; Holmboe, S.; Vildbrad, M.D.; Nielsen-Kudsk, J.E. The effects of cyclic guanylate cyclase stimulation on right ventricular hypertrophy and failure alone and in combination with phosphodiesterase-5 inhibition. J. Cardiovasc. Pharmacol. 2013, 62, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Axelsen, J.B.; Andersen, S.; Sun, X.Q.; Ringgaard, S.; Hyldebrandt, J.A.; Kurakula, K.; Goumans, M.J.; de Man, F.S.; Nielsen-Kudsk, J.E.; Bogaard, H.J.; et al. Effects of 6-mercaptopurine in pressure overload induced right heart failure. PLoS ONE 2019, 14, e0225122. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.; Schultz, J.G.; Holmboe, S.; Axelsen, J.B.; Hansen, M.S.; Lyhne, M.D.; Nielsen-Kudsk, J.E.; Andersen, A. A Pulmonary Trunk Banding Model of Pressure Overload Induced Right Ventricular Hypertrophy and Failure. J. Vis. Exp. 2018, 141, e58050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmboe, S.; Andersen, A.; Johnsen, J.; Nielsen, J.M.; Norregaard, R.; Botker, H.E.; Clapp, L.H.; Nielsen-Kudsk, J.E. Inotropic Effects of Prostacyclins on the Right Ventricle Are Abolished in Isolated Rat Hearts With Right-Ventricular Hypertrophy and Failure. J. Cardiovasc. Pharmacol. 2017, 69, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bogaard, H.J.; Natarajan, R.; Henderson, S.C.; Long, C.S.; Kraskauskas, D.; Smithson, L.; Ockaili, R.; McCord, J.M.; Voelkel, N.F. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 2009, 120, 1951–1960. [Google Scholar] [CrossRef] [Green Version]
- Swift, A.J.; Capener, D.; Hammerton, C.; Thomas, S.M.; Elliot, C.; Condliffe, R.; Wild, J.M.; Kiely, D.G. Right ventricular sex differences in patients with idiopathic pulmonary arterial hypertension characterised by magnetic resonance imaging: Pair-matched case controlled study. PLoS ONE 2015, 10, e0127415. [Google Scholar] [CrossRef]
- Bustamante-Labarta, M.; Perrone, S.; De La Fuente, R.L.; Stutzbach, P.; De La Hoz, R.P.; Torino, A.; Favaloro, R. Right atrial size and tricuspid regurgitation severity predict mortality or transplantation in primary pulmonary hypertension. J. Am. Soc. Echocardiogr. 2002, 15, 1160–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.; Vanderpool, R.R.; Avazmohammadi, R.; Lapshin, E.; Bachman, T.N.; Sacks, M.; Simon, M.A. Biomechanical and Hemodynamic Measures of Right Ventricular Diastolic Function: Translating Tissue Biomechanics to Clinical Relevance. J. Am. Heart Assoc. 2017, 6, e006084. [Google Scholar] [CrossRef] [Green Version]
- Andersen, S.; Birkmose Axelsen, J.; Ringgaard, S.; Randel Nyengaard, J.; Holm Nielsen, S.; Genovese, F.; Asser Karsdal, M.; Adler Hyldebrandt, J.; Brandt Sorensen, C.; de Man, F.S.; et al. Pressure overload induced right ventricular remodeling is not attenuated by the anti-fibrotic agent pirfenidone. Pulm. Circ. 2019, 9, 2045894019848659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axelgaard, S.; Holmboe, S.; Ringgaard, S.; Hillgaard, T.K.; Andersen, S.; Hansen, M.S.; Andersen, A.; Nielsen-Kudsk, J.E. Effects of chronic treprostinil treatment on experimental right heart hypertrophy and failure. Cardiol. Young 2017, 27, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, H.; Hirata, Y.; Yoshimi, H.; Takata, S.; Takagi, Y.; Iida, T.; Yamane, Y.; Umeda, Y.; Nishikawa, M.; Inada, M. Effects of steroid and thyroid hormones on synthesis of atrial natriuretic peptide by cultured atrial myocytes of rat. Biochem. Biophys. Res. Commun. 1987, 145, 336–343. [Google Scholar] [CrossRef]
- Matsubara, H.; Hirata, Y.; Yoshimi, H.; Takata, S.; Takagi, Y.; Yamane, Y.; Umeda, Y.; Nishikawa, M.; Inada, M. Ventricular myocytes from neonatal rats are more responsive to dexamethasone than atrial myocytes in synthesis of atrial natriuretic peptide. Biochem. Biophys. Res. Commun. 1987, 148, 1030–1038. [Google Scholar] [CrossRef]
- Hutson, D.D.; Gurrala, R.; Ogola, B.O.; Zimmerman, M.A.; Mostany, R.; Satou, R.; Lindsey, S.H. Estrogen receptor profiles across tissues from male and female Rattus norvegicus. Biol. Sex. Differ. 2019, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Pugach, E.K.; Blenck, C.L.; Dragavon, J.M.; Langer, S.J.; Leinwand, L.A. Estrogen receptor profiling and activity in cardiac myocytes. Mol. Cell Endocrinol. 2016, 431, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex. Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Andersen, S.; Nielsen-Kudsk, J.E.; Vonk Noordegraaf, A.; de Man, F.S. Right Ventricular Fibrosis. Circulation 2019, 139, 269–285. [Google Scholar] [CrossRef]
- Heger, J.; Schulz, R.; Euler, G. Molecular switches under TGFbeta signalling during progression from cardiac hypertrophy to heart failure. Br. J. Pharmacol. 2016, 173, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engebretsen, K.V.; Skardal, K.; Bjornstad, S.; Marstein, H.S.; Skrbic, B.; Sjaastad, I.; Christensen, G.; Bjornstad, J.L.; Tonnessen, T. Attenuated development of cardiac fibrosis in left ventricular pressure overload by SM16, an orally active inhibitor of ALK5. J. Mol. Cell Cardiol. 2014, 76, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Docx, C.; Holmes, A.M.; Beach, S.; Duggan, N.; England, K.; Leblanc, C.; Lebret, C.; Schindler, F.; Raza, F.; et al. Activin-like kinase 5 (ALK5) mediates abnormal proliferation of vascular smooth muscle cells from patients with familial pulmonary arterial hypertension and is involved in the progression of experimental pulmonary arterial hypertension induced by monocrotaline. Am. J. Pathol. 2009, 174, 380–389. [Google Scholar]
- Zaiman, A.L.; Podowski, M.; Medicherla, S.; Gordy, K.; Xu, F.; Zhen, L.; Shimoda, L.A.; Neptune, E.; Higgins, L.; Murphy, A.; et al. Role of the TGF-beta/Alk5 signaling pathway in monocrotaline-induced pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2008, 177, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.Y.; Yamashita, M.; Coussens, N.P.; Tang, Y.; Wang, X.; Li, C.; Deng, C.X.; Cheng, S.Y.; Zhang, Y.E. Ablation of Smurf2 reveals an inhibition in TGF-beta signalling through multiple mono-ubiquitination of Smad3. EMBO J. 2011, 30, 4777–4789. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Zhang, Z.; van Dam, H.; Zhang, L.; Zhou, F. Regulation of TGF-beta Superfamily Signaling by SMAD Mono-Ubiquitination. Cells 2014, 3, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Partovian, C.; Adnot, S.; Eddahibi, S.; Teiger, E.; Levame, M.; Dreyfus, P.; Raffestin, B.; Frelin, C. Heart and lung VEGF mRNA expression in rats with monocrotaline- or hypoxia-induced pulmonary hypertension. Am. J. Physiol. 1998, 275, H1948–H1956. [Google Scholar] [CrossRef]
- Sutendra, G.; Dromparis, P.; Paulin, R.; Zervopoulos, S.; Haromy, A.; Nagendran, J.; Michelakis, E.D. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J. Mol. Med. 2013, 91, 1315–1327. [Google Scholar] [CrossRef]
- Thenappan, T.; Chan, S.Y.; Weir, E.K. Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1322–H1331. [Google Scholar] [CrossRef]
- Dean, A.; Gregorc, T.; Docherty, C.K.; Harvey, K.Y.; Nilsen, M.; Morrell, N.W.; MacLean, M.R. Role of the Aryl Hydrocarbon Receptor in Sugen 5416-induced Experimental Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2018, 58, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Austin, E.D.; Talati, M.; Fessel, J.P.; Farber-Eger, E.H.; Brittain, E.L.; Hemnes, A.R.; Loyd, J.E.; West, J. Oestrogen inhibition reverses pulmonary arterial hypertension and associated metabolic defects. Eur. Respir. J. 2017, 50, 1602337. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Gene Name | Assay ID |
|---|---|
| B2m beta-2 microglobulin | Rn00560865_m1 |
| Col1a1 collagen, type I, alpha 1 | Rn01463848_m1 |
| Cyp1b1 cytochrome P450, family 1, subfamily b, polypeptide 1 | Rn04219389_g1 |
| ID1 inhibitor of DNA binding 1 | Rn00562985_s1 |
| ID3 inhibitor of DNA binding 3 | RN00564923_m1 |
| NPPA natriuretic peptide type A | RN00664637_g1 |
| NPPB natriuretic peptide type B | Rn00580641_m1 |
| VEGFA | RN01511602_m1 |
| Smurf 1 SMAD specific E3 ubiquitin protein ligase 1 | Rn01412801_m1 |
| Smurf 2 SMAD specific E3 ubiquitin protein ligase 2 | Rn01452783_m1 |
| Smad 2 SMAD family member 2 | Rn00569900_m1 |
| Smad 3 SMAD family member 3 | Rn00565331_m1 |
| Smad 7 SMAD family member 7 | Rn01523958_m1 |
| GPER G protein-coupled estrogen receptor 1 | Rn01643280_s1 |
| ESR 1 estrogen receptor 1 (alpha) | Rn01640372_m1 |
| ESR 2 estrogen receptor 1 (beta) | Rn00562610_m1 |
| Tgfbr1 transforming growth factor, beta receptor I | Rn00688966_m1 |
| Htr1b 5-hydroxytryptamine (serotonin) receptor 1B | Rn01637747_s1 |
| SERT Serotonin Transporter | Rn00564737_m1 |
| Bmpr2 bone morphogenetic protein receptor, type II (serine/threonine kinase) | Rn01437214_m1 |
| Cyp1a1 cytochrome P450, family 1, subfamily a, polypeptide 1 | Rn00487218_m1 |
| Fn1 fibronectin 1 | Rn00569575_m1 |
| Col3a1 collagen, type III, alpha 1 | Rn01437681_m1 |
| Tgfb1 transforming growth factor, beta 1 | Rn00572010_m1 |
| Male-Sham (n = 6) | Male-PTB (n = 10) | Female-Sham (n = 6) | Female-PTB (n = 10) | |
|---|---|---|---|---|
| Body Weight (Initial); g | 109.2 ± 3.9 | 112.5 ± 2.2 | 106.3 ± 4.8 | 106 ± 2.8 |
| Body Weight (Final); g | 398 ± 16.4 | 381.4 ± 8.3 | 245.3 ± 5.5 * | 238.2 ± 5.4 ≠ |
| Liver; g | 14.75 ± 0.89 | 13.22 ± 0.46 | 8.35 ± 0.35 * | 8.29 ± 0.29 ≠ |
| Lung; g | 1.64 ± 0.10 | 1.66 ± 0.04 | 1.35 ± 0.06 * | 1.21 ± 0.03 ≠ |
| Spleen; g | 1.03 ± 0.09 | 0.95 ± 0.08 | 0.73 ± 0.02 * | 0.69 ± 0.02 ≠ |
| Kidney; g | 2.44 ± 0.10 | 2.32 ± 0.07 | 1.71 ± 0.06 * | 1.60 ± 0.03 ≠ |
| Tibia; mm | 40.98 ± 0.29 | 40.50 ± 0.28 | 37.23 ± 0.19 * | 37 ± 0.22 ≠ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labazi, H.; Axelsen, J.B.; Hillyard, D.; Nilsen, M.; Andersen, A.; MacLean, M.R. Sex-Dependent Changes in Right Ventricular Gene Expression in Response to Pressure Overload in a Rat Model of Pulmonary Trunk Banding. Biomedicines 2020, 8, 430. https://doi.org/10.3390/biomedicines8100430
Labazi H, Axelsen JB, Hillyard D, Nilsen M, Andersen A, MacLean MR. Sex-Dependent Changes in Right Ventricular Gene Expression in Response to Pressure Overload in a Rat Model of Pulmonary Trunk Banding. Biomedicines. 2020; 8(10):430. https://doi.org/10.3390/biomedicines8100430
Chicago/Turabian StyleLabazi, Hicham, Julie Birkmose Axelsen, Dianne Hillyard, Margaret Nilsen, Asger Andersen, and Margaret R. MacLean. 2020. "Sex-Dependent Changes in Right Ventricular Gene Expression in Response to Pressure Overload in a Rat Model of Pulmonary Trunk Banding" Biomedicines 8, no. 10: 430. https://doi.org/10.3390/biomedicines8100430