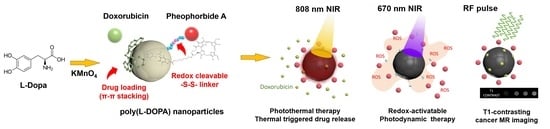
T1-Positive Mn2+-Doped Multi-Stimuli Responsive poly(L-DOPA) Nanoparticles for Photothermal and Photodynamic Combination Cancer Therapy
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. KMnO4-Oxidative Synthesis of COOH End-Capped MNPs Using L-DOPA
2.3. Cellular Redox System-Activatable Photodynamic Design of MNPs
2.4. Loading of Aromatic Cancer Drugs onto MNPs via π–π Stacking
2.5. Physicochemical Characterization of MNPs
2.6. Evaluation of Cellular Redox System-Activatable Photodynamic Functions of MNPs
2.7. Estimation of 808 nm NIR-Responsive Photothermal Functions of MNPs
2.8. Evaluation of Mn2+-Based T1-Contrast Effects of MNPs
2.9. In Vitro Photothermal Cellular Uptake Study
2.10. In Vitro Synergistic Photodynamic and Photothermal Cytotoxic Effects of MNPs
2.11. In Vivo Photodynamic and Photothermal Tumor Ablation Studies
3. Results and Discussion
3.1. Characterization of L-DOPA-Derived and COOH End-Capped MNPs
3.2. Doxorubicin and SN38 Loading on MNPs via π- π Stacking
3.3. NIR-Responsive Photothermal Effects of MNPs
3.3.1. In Vitro and in Vivo Photothermal Effects of MNPs
3.3.2. Photothermal Triggered Drug Release
3.3.3. Calculation of Photothermal Conversion Efficiency of MNPs
3.4. Redox-Responsive Dequenching of PheoA
3.5. Cellular Redox System-Activatable Photodynamic ROS Generation by MNPs
3.6. T1-Weighted MR Imaging Properties of MNPs
3.7. Enhanced Cellular Uptake of MNPs via Photothermal Effects
3.8. PDT/PTT Synergistic Cytotoxic Effects of Doxo/PheoA-MNPs
3.9. In Vivo PDT/PTT Anti-Cancer Efficacy of MNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, Q.-H.; Fu, J.-Q.; Fu, F.-M.; Zhang, J.; Wang, C. Survival and time to initiation of adjuvant chemotherapy among breast cancer patients: A systematic review and meta-analysis. Oncotarget 2017, 9, 2739–2751. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Cheng, F.; Yan, J.; Cao, J.; Luo, K.; Pu, Y.; He, B. Hierarchical nanocomposites of graphene oxide and PEGylated protoporphyrin as carriers to load doxorubicin hydrochloride for trimodal synergistic therapy. J. Mater. Chem. B 2018, 6, 4687–4696. [Google Scholar] [CrossRef] [PubMed]
- Sanvicens, N.; Marco, M.P. Multifunctional nanoparticles–properties and prospects for their use in human medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.L.L.; Thong, P.S.P.; Soo, K.C.; Kah, J.C.Y. Protein corona in drug delivery for multimodal cancer therapy in vivo. Nanoscale 2018, 10, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Luo, L.; Li, L.; He, Y.; Cao, W.; Liu, H.; Niu, K.; Gao, D. Trimodal synergistic antitumor drug delivery system based on graphene oxide. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 142–152. [Google Scholar] [CrossRef]
- Lee, J.; Jenjob, R.; Davaa, E.; Yang, S.-G. NIR-responsive ROS generating core and ROS-triggered 5′-Deoxy-5-fluorocytidine releasing shell structured water-swelling microgel for locoregional combination cancer therapy. J. Control. Release 2019, 305, 120–129. [Google Scholar] [CrossRef]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef]
- Lv, R.; Yang, P.; He, F.; Gai, S.; Yang, G.; Dai, Y.; Hou, Z.; Lin, J. An imaging-guided platform for synergistic photodynamic/photothermal/chemo-therapy with pH/temperature-responsive drug release. Biomaterials 2015, 63, 115–127. [Google Scholar] [CrossRef]
- Lv, R.; Yang, P.; He, F.; Gai, S.; Li, C.; Dai, Y.; Yang, G.; Lin, J. A Yolk-like Multifunctional Platform for Multimodal Imaging and Synergistic Therapy Triggered by a Single Near-Infrared Light. ACS Nano 2015, 9, 1630–1647. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J.; et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29, 1603864. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the Structure of Poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Mondal, S.; Thampi, A.; Puranik, M. Kinetics of Melanin Polymerization during Enzymatic and Nonenzymatic Oxidation. J. Phys. Chem. B 2018, 122, 2047–2063. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Nam, H.Y.; Min, K.H.; Kim, D.E.; Choi, J.R.; Lee, H.J.; Lee, S.C. Mussel-inspired poly(L-DOPA)-templated mineralization for calcium phosphate-assembled intracellular nanocarriers. Colloids Surf. B Biointerfaces 2017, 157, 215–222. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Zavareh, S.; Gazi, E.M.; Jamili, M. Assessment of novel core–shell Fe3O4@poly l-DOPA nanoparticles for targeted Taxol® delivery to breast tumor in a mouse model. Mater. Sci. Eng.: C 2018, 93, 1036–1043. [Google Scholar] [CrossRef]
- Zeise, L.; Murr, B.L.; Chedekel, M.R. Melanin Standard Method: Particle Description. Pigment Cell Res. 1992, 5, 132–142. [Google Scholar] [CrossRef]
- Du, C.; Qian, J.; Zhou, L.; Su, Y.; Zhang, R.; Dong, C.-M. Biopolymer–Drug Conjugate Nanotheranostics for Multimodal Imaging-Guided Synergistic Cancer Photothermal–Chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 31576–31588. [Google Scholar] [CrossRef]
- Ozlu, B.; Kabay, G.; Bocek, I.; Yilmaz, M.; Piskin, A.K.; Shim, B.S.; Mutlu, M. Controlled release of doxorubicin from polyethylene glycol functionalized melanin nanoparticles for breast cancer therapy: Part I. Production and drug release performance of the melanin nanoparticles. Int. J. Pharm. 2019, 570, 118613. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, J.; Wang, Z.; Zhou, Z.; Bai, R.; Lu, N.; Liu, Y.; Fu, X.; Jacobson, O.; Fan, W.; et al. Core–Satellite Polydopamine–Gadolinium-Metallofullerene Nanotheranostics for Multimodal Imaging Guided Combination Cancer Therapy. Adv. Mater. 2017, 29, 1701013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, X.; Guo, S.; Lin, T.; Guo, H. Highly effective photothermal chemotherapy with pH-responsive polymer-coated drug-loaded melanin-like nanoparticles. Int. J. Nanomed. 2017, 12, 1827–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, M.H.; Li, Y.; Lo, P.-C.; Lee, H.; Choi, Y. Fucoidan-Based Theranostic Nanogel for Enhancing Imaging and Photodynamic Therapy of Cancer. Nano-Micro Lett. 2020, 12, 47. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef]
- Zhang, R.; Fan, Q.; Yang, M.; Cheng, K.; Lu, X.; Zhang, L.; Huang, W.; Cheng, Z. Engineering Melanin Nanoparticles as an Efficient Drug–Delivery System for Imaging-Guided Chemotherapy. Adv. Mater. 2015, 27, 5063–5069. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yan, Y.; Such, G.K.; Liang, K.; Ochs, C.J.; Postma, A.; Caruso, F. Immobilization and Intracellular Delivery of an Anticancer Drug Using Mussel-Inspired Polydopamine Capsules. Biomacromolecules 2012, 13, 2225–2228. [Google Scholar] [CrossRef]
- Kim, B.J.; Cheong, H.; Hwang, B.H.; Cha, H.J. Mussel-Inspired Protein Nanoparticles Containing Iron(III)–DOPA Complexes for pH-Responsive Drug Delivery. Angew. Chem. 2015, 127, 7426–7430. [Google Scholar] [CrossRef]
- Li, W.-Q.; Wang, Z.; Hao, S.; He, H.; Wan, Y.; Zhu, C.; Sun, L.-P.; Cheng, G.; Zheng, S.-Y. Mitochondria-Targeting Polydopamine Nanoparticles to Deliver Doxorubicin for Overcoming Drug Resistance. ACS Appl. Mater. Interfaces 2017, 9, 16793–16802. [Google Scholar] [CrossRef]
- Tang, W.; Liu, B.; Wang, S.; Liu, T.; Fu, C.; Ren, X.; Tan, L.; Duan, W.; Meng, X. Doxorubicin-loaded ionic liquid–polydopamine nanoparticles for combined chemotherapy and microwave thermal therapy of cancer. RSC Adv. 2016, 6, 32434–32440. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, C.; Zhang, D.; Wang, Y.; Ren, X.; Ai, K.; Chen, X.; Lu, L. Targeted polydopamine nanoparticles enable photoacoustic imaging guided chemo-photothermal synergistic therapy of tumor. Acta Biomater. 2017, 47, 124–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hessel, C.M.; Pattani, V.P.; Rasch, M.; Panthani, M.G.; Koo, B.; Tunnell, J.W.; Korgel, B.A. Copper Selenide Nanocrystals for Photothermal Therapy. Nano Lett. 2011, 11, 2560–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, P.; An, R.; Zhang, P.; Yao, S.; Song, S.; Dong, L.; Xu, X.; Du, K.; Feng, J.; Zhang, H. Ultrafast Synthesis of Ultrasmall Poly(Vinylpyrrolidone)-Protected Bismuth Nanodots as a Multifunctional Theranostic Agent for In Vivo Dual-Modal CT/Photothermal-Imaging-Guided Photothermal Therapy. Adv. Funct. Mater. 2017, 27, 1702018. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Yang, X.; Tang, Y.; Han, S.; Kang, A.; Deng, H.; Chi, Y.; Zhu, D.; Lu, Y. FÖrster resonance energy transfer (FRET)-based biosensors for biological applications. Biosens. Bioelectron. 2019, 138, 111314. [Google Scholar] [CrossRef]
- Chen, N.-T.; Cheng, S.-H.; Liu, C.-P.; Souris, J.S.; Chen, C.-T.; Mou, C.-Y.; Lo, L.-W. Recent Advances in Nanoparticle-Based Förster Resonance Energy Transfer for Biosensing, Molecular Imaging and Drug Release Profiling. Int. J. Mol. Sci. 2012, 13, 16598–16623. [Google Scholar] [CrossRef]
- Dulkeith, E.; Morteani, A.C.; Niedereichholz, T.; Klar, T.A.; Feldmann, J.; Levi, S.A.; van Veggel, F.C.J.M.; Reinhoudt, D.N.; Möller, M.; Gittins, D.I. Fluorescence Quenching of Dye Molecules near Gold Nanoparticles: Radiative and Nonradiative Effects. Phys. Rev. Lett. 2002, 89, 203002. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef]
- Li, L.; Nurunnabi, M.D.; Nafiujjaman, M.D.; Lee, Y.; Huh, K.M. GSH-mediated photoactivity of pheophorbide a-conjugated heparin/gold nanoparticle for photodynamic therapy. J. Control. Release 2013, 171, 241–250. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; DeGraff, W.; Friedman, N.; Mitchell, J.B. Selective Modulation of Glutathione Levels in Human Normal versus Tumor Cells and Subsequent Differential Response to Chemotherapy Drugs. Cancer Res. 1986, 46, 2845–2848. [Google Scholar]
- Cho, S.; Park, W.; Kim, D.-H. Silica-Coated Metal Chelating-Melanin Nanoparticles as a Dual-Modal Contrast Enhancement Imaging and Therapeutic Agent. ACS Appl. Mater. Interfaces 2017, 9, 101–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, K.-Y.; Lee, J.W.; Im, G.H.; Lee, S.; Pyo, J.; Park, S.B.; Lee, J.H.; Lee, J.-K. Bio-Inspired, Melanin-Like Nanoparticles as a Highly Efficient Contrast Agent for T1-Weighted Magnetic Resonance Imaging. Biomacromolecules 2013, 14, 3491–3497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Q.; He, X.; Chen, H.; Zou, Y.; Li, Y.; Lin, K.; Cai, X.; Xiao, J.; Zhang, Q.; et al. Multifunctional melanin-like nanoparticles for bone-targeted chemo-photothermal therapy of malignant bone tumors and osteolysis. Biomaterials 2018, 183, 10–19. [Google Scholar] [CrossRef]
- Chen, Y.; Ai, K.; Liu, J.; Ren, X.; Jiang, C.; Lu, L. Polydopamine-based coordination nanocomplex for T1/T2 dual mode magnetic resonance imaging-guided chemo-photothermal synergistic therapy. Biomaterials 2016, 77, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.-H.; Wang, H.; Yang, H.; Li, Z.-L.; Zhen, L.; Xu, C.-Y. Intrinsically Mn2+-Chelated Polydopamine Nanoparticles for Simultaneous Magnetic Resonance Imaging and Photothermal Ablation of Cancer Cells. ACS Appl. Mater. Interfaces 2015, 7, 16946–16952. [Google Scholar] [CrossRef]
- Xu, W.; Sun, J.; Li, L.; Peng, X.; Zhang, R.; Wang, B. Melanin-manganese nanoparticles with ultrahigh efficient clearance in vivo for tumor-targeting T 1 magnetic resonance imaging contrast agent. Biomater. Sci. 2018, 6, 207–215. [Google Scholar] [CrossRef]
- Wu, D.; Duan, X.; Guan, Q.; Liu, J.; Yang, X.; Zhang, F.; Huang, P.; Shen, J.; Shuai, X.; Cao, Z. Mesoporous Polydopamine Carrying Manganese Carbonyl Responds to Tumor Microenvironment for Multimodal Imaging-Guided Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1900095. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, D.; Rosenkrans, Z.T.; Ehlerding, E.B.; Ni, D.; Qi, C.; Kutyreff, C.J.; Barnhart, T.E.; Engle, J.W.; Huang, P.; et al. A Melanin-Based Natural Antioxidant Defense Nanosystem for Theranostic Application in Acute Kidney Injury. Adv. Funct. Mater. 2019, 29, 1904833. [Google Scholar] [CrossRef]
- Tseng, Y.-J.; Chou, S.-W.; Shyue, J.-J.; Lin, S.-Y.; Hsiao, J.-K.; Chou, P.-T. A Versatile Theranostic Delivery Platform Integrating Magnetic Resonance Imaging/Computed Tomography, pH/cis-Diol Controlled Release, and Targeted Therapy. ACS Nano 2016, 10, 5809–5822. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.; Oldendorf , W., Jr. Basics of Magnetic Resonance Imaging; Springer Science & Business Media: New York, NY, USA, 1988; ISBN 978-0-89838-964-7. [Google Scholar]
- Fan, Q.; Cheng, K.; Hu, X.; Ma, X.; Zhang, R.; Yang, M.; Lu, X.; Xing, L.; Huang, W.; Gambhir, S.S.; et al. Transferring Biomarker into Molecular Probe: Melanin Nanoparticle as a Naturally Active Platform for Multimodality Imaging. J. Am. Chem. Soc. 2014, 136, 15185–15194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Xu, W.; Li, L.; Fan, B.; Peng, X.; Qu, B.; Wang, L.; Li, T.; Li, S.; Zhang, R. Ultrasmall endogenous biopolymer nanoparticles for magnetic resonance/photoacoustic dual-modal imaging-guided photothermal therapy. Nanoscale 2018, 10, 10584–10595. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Gong, H.; Gao, M.; Zhu, W.; Sun, X.; Feng, L.; Fu, T.; Li, Y.; Liu, Z. Polydopamine Nanoparticles as a Versatile Molecular Loading Platform to Enable Imaging-guided Cancer Combination Therapy. Theranostics 2016, 6, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wires Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Poinard, B.; Neo, S.Z.Y.; Yeo, E.L.L.; Heng, H.P.S.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Nanoparticles Enhance Drug Release for Combined Photodynamic and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21125–21136. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, Y.; Duan, Y. Application of polydopamine in tumor targeted drug delivery system and its drug release behavior. J. Control. Release 2018, 290, 56–74. [Google Scholar] [CrossRef]
- Araújo, M.; Viveiros, R.; Correia, T.R.; Correia, I.J.; Bonifácio, V.D.B.; Casimiro, T.; Aguiar-Ricardo, A. Natural melanin: A potential pH-responsive drug release device. Int. J. Pharm. 2014, 469, 140–145. [Google Scholar] [CrossRef]
- Ho, C.-C.; Ding, S.-J. The pH-controlled nanoparticles size of polydopamine for anti-cancer drug delivery. J. Mater. Sci. Mater. Med. 2013, 24, 2381–2390. [Google Scholar] [CrossRef]







| Elements | ||||||
|---|---|---|---|---|---|---|
| C | O | Mn | ||||
| wt% | at% | wt% | at% | wt% | at% | |
| Polydopamine NPs (PDA NPs) | 9.40 | 12.15 | 90.52 | 87.53 | 0.08 | 0.02 |
| Poly(L-DOPA) NPs (MNPs) | 8.07 | 11.42 | 63.77 | 67.76 | 28.16 | 20.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Baskaran, R.; Ozlu, B.; Davaa, E.; Kim, J.J.; Shim, B.S.; Yang, S.-G. T1-Positive Mn2+-Doped Multi-Stimuli Responsive poly(L-DOPA) Nanoparticles for Photothermal and Photodynamic Combination Cancer Therapy. Biomedicines 2020, 8, 417. https://doi.org/10.3390/biomedicines8100417
Kang S, Baskaran R, Ozlu B, Davaa E, Kim JJ, Shim BS, Yang S-G. T1-Positive Mn2+-Doped Multi-Stimuli Responsive poly(L-DOPA) Nanoparticles for Photothermal and Photodynamic Combination Cancer Therapy. Biomedicines. 2020; 8(10):417. https://doi.org/10.3390/biomedicines8100417
Chicago/Turabian StyleKang, Sumin, Rengarajan Baskaran, Busra Ozlu, Enkhzaya Davaa, Jung Joo Kim, Bong Sup Shim, and Su-Geun Yang. 2020. "T1-Positive Mn2+-Doped Multi-Stimuli Responsive poly(L-DOPA) Nanoparticles for Photothermal and Photodynamic Combination Cancer Therapy" Biomedicines 8, no. 10: 417. https://doi.org/10.3390/biomedicines8100417