Cystatin from Filarial Parasites Suppress the Clinical Symptoms and Pathology of Experimentally Induced Colitis in Mice by Inducing T-Regulatory Cells, B1-Cells, and Alternatively Activated Macrophages
Abstract
:1. Introduction
2. Results
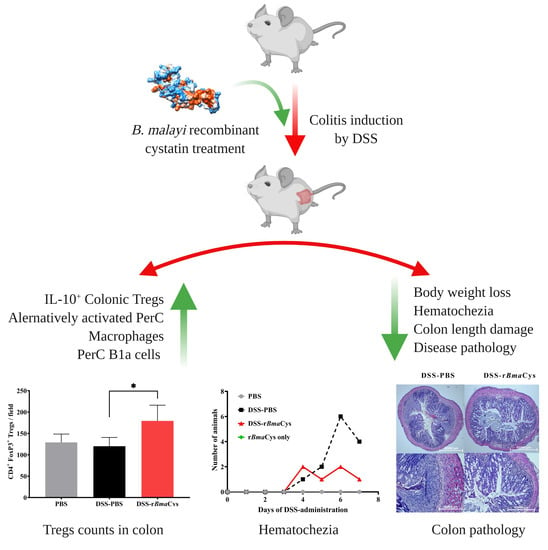
2.1. rBmaCys-Treatment Attenuated DSS-Induced Clinical Signs of Colitis in Mice
2.2. rBmaCys-Treatment Significantly Decreased the Expression of Pro-Inflammatory Cytokines in the Colon Tissues
2.3. IL-10+ Tregulatory Cells (Tregs) were increased in the Colon of rBmaCys-Treated Colitis Mice
2.4. Peritoneal Macrophages from rBmaCys-Treated Colitis Mice Produced Lesser Amounts of Nitric Oxide and Th1 Cytokines
2.5. IgM+B1a Cells were increased in the Peritoneal Cavity of rBmaCys-Treated Colitis Mice
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of rBmaCys
4.2. Animals
4.3. DSS-Induced Colitis Model
4.4. Evaluation of Colitis in the Mice Treated with rBmaCys
4.5. qPCR Analysis to Determine Relative Cytokine mRNA Expression in the Colon Tissue and in Peritoneal Macrophages
4.6. Levels of Nitric Oxide in the Culture Supernatants of Peritoneal Macrophages
4.7. Flow Cytometric Analysis of Treg and B-1 Cells
4.7.1. Analysis of the LPMCs
4.7.2. Analysis of Spleens and MLNs
4.7.3. Analysis of Peritoneal B1 Cells
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Kahl, J.; Brattig, N.; Liebau, E. The Untapped Pharmacopeic Potential of Helminths. Trends Parasitol. 2018. [Google Scholar] [CrossRef]
- Weinstock, J.V.; Elliott, D.E. Helminth infections decrease host susceptibility to immune-mediated diseases. J. Immunol. 2014, 193, 3239–3247. [Google Scholar] [CrossRef] [PubMed]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Nikolopoulos, G.K.; Peyrin-Biroulet, L.; Danese, S. Biologic Therapies and Risk of Infection and Malignancy in Patients with Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1385–1397 e1310. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, T.B.; Giacomin, P.R.; Loukas, A.; Mulvenna, J.P.; Clark, R.J.; Miles, J.J. Helminth Immunomodulation in Autoimmune Disease. Front. Immunol. 2017, 8, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakeri, A.; Hansen, E.P.; Andersen, S.D.; Williams, A.R.; Nejsum, P. Immunomodulation by Helminths: Intracellular Pathways and Extracellular Vesicles. Front. Immunol 2018, 9, 2349. [Google Scholar] [CrossRef]
- Chen, L.; He, B.; Hou, W.; He, L. Cysteine protease inhibitor of Schistosoma japonicum-A parasite-derived negative immunoregulatory factor. Parasitol. Res. 2017, 116, 901–908. [Google Scholar] [CrossRef]
- Coronado, S.; Barrios, L.; Zakzuk, J.; Regino, R.; Ahumada, V.; Franco, L.; Ocampo, Y.; Caraballo, L. A recombinant cystatin from Ascaris lumbricoides attenuates inflammation of DSS-induced colitis. Parasite Immunol. 2017, 39. [Google Scholar] [CrossRef]
- Khatri, V.; Amdare, N.; Tarnekar, A.; Goswami, K.; Reddy, M.V. Brugia malayi cystatin therapeutically ameliorates dextran sulfate sodium-induced colitis in mice. J. Dig. Dis. 2015, 16, 585–594. [Google Scholar] [CrossRef]
- Jang, S.W.; Cho, M.K.; Park, M.K.; Kang, S.A.; Na, B.K.; Ahn, S.C.; Kim, D.H.; Yu, H.S. Parasitic helminth cystatin inhibits DSS-induced intestinal inflammation via IL-10+F4/80+ macrophage recruitment. Korean J. Parasitol. 2011, 49, 245–254. [Google Scholar] [CrossRef]
- Ziegler, T.; Rausch, S.; Steinfelder, S.; Klotz, C.; Hepworth, M.R.; Kuhl, A.A.; Burda, P.C.; Lucius, R.; Hartmann, S. A novel regulatory macrophage induced by a helminth molecule instructs IL-10 in CD4+ T cells and protects against mucosal inflammation. J. Immunol. 2015, 194, 1555–1564. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y.; Yang, X.; Wang, X.; Yan, K.; Zhong, Z.; Wang, X.; Xu, Y.; Zhang, Y.; Liu, F.; et al. Therapeutic potential of recombinant cystatin from Schistosoma japonicum in TNBS-induced experimental colitis of mice. Parasit. Vectors 2016, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Roig, J.; Saiz, M.L.; Galiano, A.; Trelis, M.; Cantalapiedra, F.; Monteagudo, C.; Giner, E.; Giner, R.M.; Recio, M.C.; Bernal, D.; et al. Extracellular Vesicles From the Helminth Fasciola hepatica Prevent DSS-Induced Acute Ulcerative Colitis in a T-Lymphocyte Independent Mode. Front. Microbiol. 2018, 9, 1036. [Google Scholar] [CrossRef] [PubMed]
- Togre, N.; Bhoj, P.; Goswami, K.; Tarnekar, A.; Patil, M.; Shende, M. Human filarial proteins attenuate chronic colitis in an experimental mouse model. Parasite Immunol. 2018, 40. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin. Microbiol. Rev. 2002, 15, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Bressenot, A.; Salleron, J.; Bastien, C.; Danese, S.; Boulagnon-Rombi, C.; Peyrin-Biroulet, L. Comparing histological activity indexes in UC. Gut 2015, 64, 1412–1418. [Google Scholar] [CrossRef]
- Griffin, G.K.; Newton, G.; Tarrio, M.L.; Bu, D.X.; Maganto-Garcia, E.; Azcutia, V.; Alcaide, P.; Grabie, N.; Luscinskas, F.W.; Croce, K.J.; et al. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J. Immunol. 2012, 188, 6287–6299. [Google Scholar] [CrossRef]
- Tang, C.; Kakuta, S.; Shimizu, K.; Kadoki, M.; Kamiya, T.; Shimazu, T.; Kubo, S.; Saijo, S.; Ishigame, H.; Nakae, S.; et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat. Immunol. 2018, 19, 755–765. [Google Scholar] [CrossRef]
- Aguiar, S.L.F.; Miranda, M.C.G.; Guimaraes, M.A.F.; Santiago, H.C.; Queiroz, C.P.; Cunha, P.D.S.; Cara, D.C.; Foureaux, G.; Ferreira, A.J.; Cardoso, V.N.; et al. High-Salt Diet Induces IL-17-Dependent Gut Inflammation and Exacerbates Colitis in Mice. Front. Immunol. 2017, 8, 1969. [Google Scholar] [CrossRef]
- Strober, W. Impact of the gut microbiome on mucosal inflammation. Trends Immunol. 2013, 34, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Tait Wojno, E.D.; Artis, D. Innate lymphoid cells: Balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe 2012, 12, 445–457. [Google Scholar] [CrossRef]
- Spits, H.; Di Santo, J.P. The expanding family of innate lymphoid cells: Regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 2011, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Robinette, M.L.; Colonna, M. Immune modules shared by innate lymphoid cells and T cells. J. Allergy Clin. Immunol. 2016, 138, 1243–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido-Mesa, N.; Schroeder, J.H.; Stolarczyk, E.; Gallagher, A.L.; Lo, J.W.; Bailey, C.; Campbell, L.; Sexl, V.; MacDonald, T.T.; Howard, J.K.; et al. T-bet controls intestinal mucosa immune responses via repression of type 2 innate lymphoid cell function. Mucosal Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Izcue, A.; Coombes, J.L.; Powrie, F. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 2009, 27, 313–338. [Google Scholar] [CrossRef]
- Campbell, D.J.; Koch, M.A. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 2011, 11, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Schnoeller, C.; Rausch, S.; Pillai, S.; Avagyan, A.; Wittig, B.M.; Loddenkemper, C.; Hamann, A.; Hamelmann, E.; Lucius, R.; Hartmann, S. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J. Immunol. 2008, 180, 4265–4272. [Google Scholar] [CrossRef]
- Smith, H.; Forman, R.; Mair, I.; Else, K.J. Interactions of helminths with macrophages: Therapeutic potential for inflammatory intestinal disease. Expert Rev. Gastroenterol. Hepatol. 2018, 1–10. [Google Scholar] [CrossRef]
- Su, C.; Su, L.; Li, Y.; Long, S.R.; Chang, J.; Zhang, W.; Walker, W.A.; Xavier, R.J.; Cherayil, B.J.; Shi, H.N. Helminth-induced alterations of the gut microbiota exacerbate bacterial colitis. Mucosal Immunol. 2018, 11, 144–157. [Google Scholar] [CrossRef]
- Al-Qaoud, K.M.; Fleischer, B.; Hoerauf, A. The Xid defect imparts susceptibility to experimental murine filariosis--association with a lack of antibody and IL-10 production by B cells in response to phosphorylcholine. Int. Immunol. 1998, 10, 17–25. [Google Scholar] [CrossRef]
- Smith, P.; Mangan, N.E.; Walsh, C.M.; Fallon, R.E.; McKenzie, A.N.; van Rooijen, N.; Fallon, P.G. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J. Immunol. 2007, 178, 4557–4566. [Google Scholar] [CrossRef]
- Fillatreau, S.; Sweenie, C.H.; McGeachy, M.J.; Gray, D.; Anderton, S.M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002, 3, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Gray, D.; Mushtaq, N.; Londei, M. Prevention of arthritis by interleukin 10-producing B cells. J. Exp. Med. 2003, 197, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, A.; Mizoguchi, E.; Takedatsu, H.; Blumberg, R.S.; Bhan, A.K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 2002, 16, 219–230. [Google Scholar] [CrossRef]
- Wolf, S.D.; Dittel, B.N.; Hardardottir, F.; Janeway, C.A., Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J. Exp. Med. 1996, 184, 2271–2278. [Google Scholar] [CrossRef]
- Smits, H.H.; Hammad, H.; van Nimwegen, M.; Soullie, T.; Willart, M.A.; Lievers, E.; Kadouch, J.; Kool, M.; Kos-van Oosterhoud, J.; Deelder, A.M.; et al. Protective effect of Schistosoma mansoni infection on allergic airway inflammation depends on the intensity and chronicity of infection. J. Allergy Clin. Immunol. 2007, 120, 932–940. [Google Scholar] [CrossRef]
- Harnett, W.; McInnes, I.B.; Harnett, M.M. ES-62, a filarial nematode-derived immunomodulator with anti-inflammatory potential. Immunol. Lett. 2004, 94, 27–33. [Google Scholar] [CrossRef]
- Aziz, M.; Holodick, N.E.; Rothstein, T.L.; Wang, P. The role of B-1 cells in inflammation. Immunol. Res. 2015, 63, 153–166. [Google Scholar] [CrossRef]
- Rodgers, D.T.; Pineda, M.A.; McGrath, M.A.; Al-Riyami, L.; Harnett, W.; Harnett, M.M. Protection against collagen-induced arthritis in mice afforded by the parasitic worm product, ES-62, is associated with restoration of the levels of interleukin-10-producing B cells and reduced plasma cell infiltration of the joints. Immunology 2014, 141, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Wilson, E.H.; Katz, E.; Goodridge, H.S.; Harnett, M.M.; Harnett, W. In vivo activation of murine peritoneal B1 cells by the filarial nematode phosphorylcholine-containing glycoprotein ES-62. Parasite Immunol. 2003, 25, 463–466. [Google Scholar] [CrossRef]
- Kawahara, T.; Ohdan, H.; Zhao, G.; Yang, Y.G.; Sykes, M. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J. Immunol. 2003, 171, 5406–5414. [Google Scholar] [CrossRef]
- Kiyohara, H.; Sujino, T.; Teratani, T.; Miyamoto, K.; Arai, M.M.; Nomura, E.; Harada, Y.; Aoki, R.; Koda, Y.; Mikami, Y.; et al. Toll-Like Receptor 7 Agonist-Induced Dermatitis Causes Severe Dextran Sulfate Sodium Colitis by Altering the Gut Microbiome and Immune Cells. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Preisker, S.; Brethack, A.K.; Bokemeyer, A.; Bettenworth, D.; Sina, C.; Derer, S. Crohn’s Disease Patients in Remission Display an Enhanced Intestinal IgM+ B Cell Count in Concert with a Strong Activation of the Intestinal Complement System. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Ling, K.L.; Pratap, S.E.; Bates, G.J.; Singh, B.; Mortensen, N.J.; George, B.D.; Warren, B.F.; Piris, J.; Roncador, G.; Fox, S.B.; et al. Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer Immun. 2007, 7, 7. [Google Scholar]
- Weigmann, B.; Tubbe, I.; Seidel, D.; Nicolaev, A.; Becker, C.; Neurath, M.F. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2007, 2, 2307–2311. [Google Scholar] [CrossRef]






| Groups | Histopathological Score |
|---|---|
| (% Change with Respect to DSS-PBS Group) | |
| PBS | No change |
| DSS-PBS | 100 |
| DSS- rBmaCys | 75.4 |
| rBmaCys only | No Change |
| Sections of colon tissues from each animal were fixed, paraffin-embedded and H&E stained. Each section was scored for pre-defined histopathological gradations by a sample blinded independent histopathologist. n = 8–10 mice per group. The variations within the groups were not-significant as per the One-Way ANOVA followed by Tukey’s post-hoc test. | |
| Scoring Parameters | Score |
| Inflammation extent | |
| None | 0 |
| Mucosa | 1 |
| Mucosa and submucosa | 2 |
| Transmural | 3 |
| Inflammation severity | |
| None | 0 |
| Mild | 1 |
| Moderate | 2 |
| Severe | 3 |
| Crypt damage | |
| None | 0 |
| Superficial 1/3 damage | 1 |
| Superficial 2/3 damage | 2 |
| Patchy crypt lost; surface epithelium present | 3 |
| Crypts & surface epithelium lost | 4 |
| Colon wall thickening | |
| None | 0 |
| Mild | 1 |
| Moderate | 2 |
| Marked increase | 3 |
| Leukocyte infiltration | |
| Normal | 0 |
| Slight increase | 1 |
| Moderate increase | 2 |
| Marked increase | 3 |
| Lamina propria mononuclear cells | |
| Normal | 0 |
| Slight increase | 1 |
| Moderate increase | 2 |
| Marked increase | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisht, N.; Khatri, V.; Chauhan, N.; Kalyanasundaram, R. Cystatin from Filarial Parasites Suppress the Clinical Symptoms and Pathology of Experimentally Induced Colitis in Mice by Inducing T-Regulatory Cells, B1-Cells, and Alternatively Activated Macrophages. Biomedicines 2019, 7, 85. https://doi.org/10.3390/biomedicines7040085
Bisht N, Khatri V, Chauhan N, Kalyanasundaram R. Cystatin from Filarial Parasites Suppress the Clinical Symptoms and Pathology of Experimentally Induced Colitis in Mice by Inducing T-Regulatory Cells, B1-Cells, and Alternatively Activated Macrophages. Biomedicines. 2019; 7(4):85. https://doi.org/10.3390/biomedicines7040085
Chicago/Turabian StyleBisht, Nalini, Vishal Khatri, Nikhil Chauhan, and Ramaswamy Kalyanasundaram. 2019. "Cystatin from Filarial Parasites Suppress the Clinical Symptoms and Pathology of Experimentally Induced Colitis in Mice by Inducing T-Regulatory Cells, B1-Cells, and Alternatively Activated Macrophages" Biomedicines 7, no. 4: 85. https://doi.org/10.3390/biomedicines7040085