The Role of Wnt and R-spondin in the Stomach During Health and Disease
Abstract
:1. Introduction
2. Wnt and R-spondin Signaling
Potentiation of Wnt Signaling by R-spondin
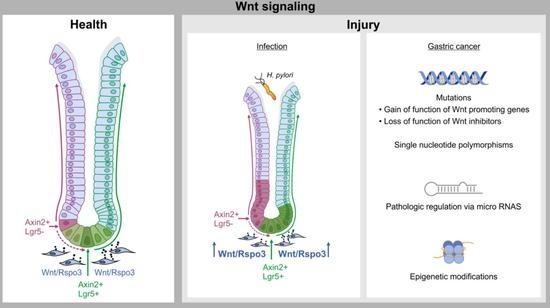
3. Wnt and R-spondin Signaling and Gastric Gland Homeostasis
4. From Healthy Tissue to Cancer: Link Between Damage, Wnt Signaling and Cancer
5. Wnt Signaling in Gastric Cancer
6. Aberrant Wnt Signaling and Its Implications for Prognosis
7. Relevance of Wnt Signaling for Cancer Therapy
8. Conclusions and Further Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nusse, R.; Varmus, H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome.pdf. Cell 1982, 31, 99–109. [Google Scholar] [CrossRef]
- Rijsewijk, F.; Schuermann, M.; Wagenaar, E.; Parren, P.; Welgel, D.; Nusse, R. The Drosophila homology of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell 1987, 50, 649–657. [Google Scholar] [CrossRef]
- Baker, N.E. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: The spatial distribution of a transcript in embryos. EMBO J. 1987, 6, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Nüsslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Najdi, R.; Proffitt, K.; Sprowl, S.; Kaur, S.; Yu, J.; Covey, T.M.; Virshup, D.M.; Waterman, M.L. A uniform human Wnt expression library reveals a shared secretory pathway and unique signaling activities. Differentiation 2012, 84, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Katoh, M.; Katoh, M. Molecular genetics and targeted therapy of WNT-related human diseases (Review). Int. J. Mol. Med. 2017, 40, 587–606. [Google Scholar] [CrossRef] [Green Version]
- Kishida, S.; Yamamoto, H.; Kikuchi, A. Wnt-3a and Dvl Induce Neurite Retraction by Activating Rho-Associated Kinase. Mol. Cell. Biol. 2004, 24, 4487–4501. [Google Scholar] [CrossRef] [Green Version]
- Mikels, A.J.; Nusse, R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006, 4, e115. [Google Scholar] [CrossRef]
- Leushacke, M.; Tan, S.H.; Wong, A.; Swathi, Y.; Hajamohideen, A.; Tan, L.T.; Goh, J.; Wong, E.; Denil, S.; Murakami, K.; et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat. Cell Biol. 2017, 19, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiurillo, M.A. Role of the Wnt/b-catenin pathway in gastric cancer: An indepth literature review. World J. Exp. Med. 2015, 5, 84–102. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, S.; Ridgway, R.A.; Cordero, J.; Athineos, D.; Salgueiro, P.; Poulsom, R.; Neumann, J.; Jung, A.; Patel, S.; Woodgett, J.; et al. Acute WNT signalling activation perturbs differentiation within the adult stomach and rapidly leads to tumour formation. Oncogene 2013, 32, 2048–2057. [Google Scholar] [CrossRef]
- Sigal, M.; Logan, C.Y.; Kapalczynska, M.; Mollenkopf, H.J.; Berger, H.; Wiedenmann, B.; Nusse, R.; Amieva, M.R.; Meyer, T.F. Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature 2017, 548, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Stamos, J.L.; Chu, M.L.; Enos, M.D.; Shah, N.; Weis, W.I. Structural basis of GSK-3 inhibition by N-terminal phosphorylation and by the Wnt receptor LRP6. Elife 2014, 3, e01998. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef] [PubMed]
- Schuijers, J.; Clevers, H. Adult mammalian stem cells: The role of Wnt, Lgr5 and R-spondins. EMBO J. 2012, 31, 2685–2696. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Huch, M.; Kujala, P.; van de Wetering, M.; Snippert, H.J.; van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef] [PubMed]
- De Lau, W.; Peng, W.C.; Gros, P.; Clevers, H. The R-spondin/Lgr5/Rnf43 module: Regulator of Wnt signal strength. Genes Dev. 2014, 28, 305–316. [Google Scholar] [CrossRef]
- Jin, Y.R.; Yoon, J.K. The R-spondin family of proteins: Emerging regulators of WNT signaling. Int. J. Biochem. Cell Biol. 2012, 44, 2278–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, H.X.; Xie, Y.; Zhang, Y.; Charlat, O.; Oster, E.; Avello, M.; Lei, H.; Mickanin, C.; Liu, D.; Ruffner, H.; et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012, 485, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Spit, M.; Jordens, I.; Low, T.Y.; Stange, D.E.; van de Wetering, M.; van Es, J.H.; Mohammed, S.; Heck, A.J.; Maurice, M.M.; et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 2012, 488, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Yokota, C.; Semenov, M.V.; Doble, B.; Woodgett, J.; He, X. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J. Biol. Chem. 2007, 282, 15903–15911. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cai, A.; Xi, H.; Li, J.; Xu, W.; Zhang, Y.; Zhang, K.; Cui, J.; Wu, X.; Wei, B.; et al. Ring finger protein 43 associates with gastric cancer progression and attenuates the stemness of gastric cancer stem-like cells via the Wnt-beta/catenin signaling pathway. Stem Cell Res. Ther. 2017, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.S.; Janda, C.Y.; Chang, J.; Zheng, G.X.Y.; Larkin, K.A.; Luca, V.C.; Chia, L.A.; Mah, A.T.; Han, A.; Terry, J.M.; et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5(+) intestinal stem-cell self-renewal. Nature 2017, 545, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Sigal, M.; Rothenberg, M.E.; Logan, C.Y.; Lee, J.Y.; Honaker, R.W.; Cooper, R.L.; Passarelli, B.; Camorlinga, M.; Bouley, D.M.; Alvarez, G.; et al. Helicobacter pylori Activates and Expands Lgr5(+) Stem Cells Through Direct Colonization of the Gastric Glands. Gastroenterology 2015, 148, 1392–1404. [Google Scholar] [CrossRef]
- Hata, M.; Hayakawa, Y.; Koike, K. Gastric Stem Cell and Cellular Origin of Cancer. Biomedicines 2018, 6, 100. [Google Scholar] [CrossRef]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Stange, D.E.; Koo, B.K.; Huch, M.; Sibbel, G.; Basak, O.; Lyubimova, A.; Kujala, P.; Bartfeld, S.; Koster, J.; Geahlen, J.H.; et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 2013, 155, 357–368. [Google Scholar] [CrossRef]
- Fafilek, B.; Krausova, M.; Vojtechova, M.; Pospichalova, V.; Tumova, L.; Sloncova, E.; Huranova, M.; Stancikova, J.; Hlavata, A.; Svec, J.; et al. Troy, a tumor necrosis factor receptor family member, interacts with lgr5 to inhibit wnt signaling in intestinal stem cells. Gastroenterology 2013, 144, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Ariyama, H.; Stancikova, J.; Sakitani, K.; Asfaha, S.; Renz, B.W.; Dubeykovskaya, Z.A.; Shibata, W.; Wang, H.; Westphalen, C.B.; et al. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell 2015, 28, 800–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, Liver Flukes and Helicobater pylori; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 1994; Volume 61, pp. 1–241. [Google Scholar]
- Vogiatzi, P.; Cassone, M.; Luzzi, I.; Lucchetti, C.; Otvos, L., Jr.; Giordano, A. Helicobacter pylori as a class I carcinogen: Physiopathology and management strategies. J. Cell Biochem. 2007, 102, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Bessede, E.; Dubus, P.; Megraud, F.; Varon, C. Helicobacter pylori infection and stem cells at the origin of gastric cancer. Oncogene 2015, 34, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Amieva, M.R.; El-Omar, E.M. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology 2008, 134, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Murata-Kamiya, N.; Kurashima, Y.; Teishikata, Y.; Yamahashi, Y.; Saito, Y.; Higashi, H.; Aburatani, H.; Akiyama, T.; Peek, R.M., Jr.; Azuma, T.; et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene 2007, 26, 4617–4626. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, N.; Chai, N.; Liu, X.; Jiang, H.; Wu, Q.; Li, Q. Helicobacter pylori promotes angiogenesis depending on Wnt/beta-catenin-mediated vascular endothelial growth factor via the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer 2016, 16, 321. [Google Scholar] [CrossRef]
- Lee, D.G.; Kim, H.S.; Lee, Y.S.; Kim, S.; Cha, S.Y.; Ota, I.; Kim, N.H.; Cha, Y.H.; Yang, D.H.; Lee, Y.; et al. Helicobacter pylori CagA promotes Snail-mediated epithelial-mesenchymal transition by reducing GSK-3 activity. Nat. Commun. 2014, 5, 4423. [Google Scholar] [CrossRef]
- Neal, J.T.; Peterson, T.S.; Kent, M.L.; Guillemin, K.H. pylori virulence factor CagA increases intestinal cell proliferation by Wnt pathway activation in a transgenic zebrafish model. Dis. Model. Mech. 2013, 6, 802–810. [Google Scholar] [CrossRef]
- Yong, X.; Tang, B.; Xiao, Y.F.; Xie, R.; Qin, Y.; Luo, G.; Hu, C.J.; Dong, H.; Yang, S.M. Helicobacter pylori upregulates Nanog and Oct4 via Wnt/beta-catenin signaling pathway to promote cancer stem cell-like properties in human gastric cancer. Cancer Lett. 2016, 374, 292–303. [Google Scholar] [CrossRef]
- Bartfeld, S.; Bayram, T.; van de Wetering, M.; Huch, M.; Begthel, H.; Kujala, P.; Vries, R.; Peters, P.J.; Clevers, H. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 2015, 148, 126–136.e126. [Google Scholar] [CrossRef] [PubMed]
- Boccellato, F.; Woelffling, S.; Imai-Matsushima, A.; Sanchez, G.; Goosmann, C.; Schmid, M.; Berger, H.; Morey, P.; Denecke, C.; Ordemann, J.; et al. Polarised epithelial monolayers of the gastric mucosa reveal insights into mucosal homeostasis and defence against infection. Gut 2018, 68, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Clements, W.M.; Wan, J.; Sarnaik, A.; Kim, O.J.; MacDonald, J.; Fenoglio-Preiser, C.; Groden, J.; Lowy, A.M. b-Catenin Mutation is a Frequent Cause of Wnt Pathway Activation in Gastric Cancer. Cancer Res. 2002, 62, 3503–3506. [Google Scholar] [PubMed]
- Zhang, H.; Xue, Y. Wnt pathway is involved in advanced gastric cancer. Hepatogastroenterology 2008, 55, 1126–1130. [Google Scholar] [PubMed]
- Mao, J.; Fan, S.; Ma, W.; Fan, P.; Wang, B.; Zhang, J.; Wang, H.; Tang, B.; Zhang, Q.; Yu, X.; et al. Roles of wnt/beta-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014, 5, e1039. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Kirikoshi, H.; Terasaki, H.; Shiokawa, K. WNT2B2 mRNA, up-regulated in primary gastric cancer, is a positive regulator of the WNT- beta-catenin-TCF signaling pathway. Biochem. Biophys. Res. Commun. 2001, 289, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Mine, T.; Katoh, M. Frequent up-regulation of Wnt5A mRNA in primary gastric cancer. Int. J. Mol. Med. 2002, 9, 515–519. [Google Scholar] [CrossRef]
- Kurayoshi, M.; Oue, N.; Yamamoto, H.; Kishida, M.; Inoue, A.; Asahara, T.; Yasui, W.; Kikuchi, A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006, 66, 10439–10448. [Google Scholar] [CrossRef]
- Yuan, G.; Regel, I.; Lian, F.; Friedrich, T.; Hitkova, I.; Hofheinz, R.D.; Strobel, P.; Langer, R.; Keller, G.; Rocken, C.; et al. WNT6 is a novel target gene of caveolin-1 promoting chemoresistance to epirubicin in human gastric cancer cells. Oncogene 2013, 32, 375–387. [Google Scholar] [CrossRef]
- Kirikoshi, H.; Sekihara, H.; Katoh, M. Up-regulation of WNT10A by tumor necrosis factor alpha and Helicobacter pylori in gastric cancer. Int. J. Oncol. 2001, 19, 533–536. [Google Scholar]
- Ebert, M.P.A.; Fei, G.; Kahmann, S.; Müller, O.; Yu, J.; Sung, J.J.Y.; Malfertheiner, P. Increased β-catenin mRNA levels and mutational alterations of the APC and β-catenin gene are present in intestinal-type gastric cancer. Carcinogenesis 2002, 23, 87–91. [Google Scholar] [CrossRef]
- Anastas, J.N.; Moon, R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 2013, 13, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Min, B.H.; Hwang, J.; Kim, N.K.; Park, G.; Kang, S.Y.; Ahn, S.; Ahn, S.; Ha, S.Y.; Lee, Y.K.; Kushima, R.; et al. Dysregulated Wnt signalling and recurrent mutations of the tumour suppressor RNF43 in early gastric carcinogenesis. J. Pathol. 2016, 240, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Rhyu, M.-G.; Park, W.-S.; Jung, Y.-J.; Choi, S.-W.; Meltzer, S.J. Allelic Deletions of MCC/APC and p53 Are Frequent Late Events in Human Gastric Carcinogenesis. Gastroenterology 1994, 106, 1584–1588. [Google Scholar] [CrossRef]
- Pan, K.-F.; Liu, W.-G.; Zhang, L.; You, W.-C.; Lu, Y.-Y. Mutations in components of the Wnt signaling pathway in gastric cancer. World J. Gastroenterol. 2008, 14, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Qin, H.Z.; Xi, H.Q.; Wei, B.; Xia, S.Y.; Chen, L. RNF43 Inhibits Cancer Cell Proliferation and Could be a Potential Prognostic Factor for Human Gastric Carcinoma. Cell. Physiol. Biochem. 2015, 36, 1835–1846. [Google Scholar] [CrossRef]
- Ksiaa, F.; Ziadi, S.; Amara, K.; Korbi, S.; Trimeche, M. Biological significance of promoter hypermethylation of tumor-related genes in patients with gastric carcinoma. Clin. Chim. Acta 2009, 404, 128–133. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, W.; Chen, Z.; Kuang, G.; Yang, Z.; Dong, Z. Hypermethylation and aberrant expression of Wnt-antagonist family genes in gastric cardia adenocarcinoma. Neoplasma 2011, 58, 110–117. [Google Scholar] [CrossRef]
- Yu, J.; Tao, Q.; Cheng, Y.Y.; Lee, K.Y.; Ng, S.S.; Cheung, K.F.; Tian, L.; Rha, S.Y.; Neumann, U.; Rocken, C.; et al. Promoter methylation of the Wnt/beta-catenin signaling antagonist Dkk-3 is associated with poor survival in gastric cancer. Cancer 2009, 115, 49–60. [Google Scholar] [CrossRef]
- Zhao, C.-H.; Bu, X.-M.; Zhang, N. Hypermethylation and aberrant expression of Wnt antagonist secreted frizzled-related protein 1 in gastric cancer. World J. Gastroenterol. 2007, 13, 2214–2217. [Google Scholar] [CrossRef]
- Shrestha, S.; Hsu, S.D.; Huang, W.Y.; Huang, H.Y.; Chen, W.; Weng, S.L.; Huang, H.D. A systematic review of microRNA expression profiling studies in human gastric cancer. Cancer Med. 2014, 3, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Volinia, S.; Okumura, H.; Shimizu, M.; Taccioli, C.; Rossi, S.; Alder, H.; Liu, C.-G.; Oue, N.; Yasui, W.; et al. Relation between microRNA expression and progression and prognosis of gastric cancer: A microRNA expression analysis. Lancet Oncol. 2010, 11, 136–146. [Google Scholar] [CrossRef]
- Tchernitsa, O.; Kasajima, A.; Schafer, R.; Kuban, R.J.; Ungethum, U.; Gyorffy, B.; Neumann, U.; Simon, E.; Weichert, W.; Ebert, M.P.; et al. Systematic evaluation of the miRNA-ome and its downstream effects on mRNA expression identifies gastric cancer progression. J. Pathol. 2010, 222, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tian, Y.; Wu, D.; Zhu, H.; Luo, D.; Gong, W.; Zhou, Y.; Zhou, J.; Zhang, Z. Genetic variation of CTNNB1 gene is associated with susceptibility and prognosis of gastric cancer in a Chinese population. Mutagenesis 2012, 27, 623–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.C.; Xu, X.Y.; Xia, P.; Yu, M.; Takahashi, H.; Takano, Y. Involvement of inactive GSK3beta overexpression in tumorigenesis and progression of gastric carcinomas. Hum. Pathol. 2010, 41, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Neumeyer, V.; Vieth, M.; Gerhard, M.; Mejias-Luque, R. Mutated Rnf43 Aggravates Helicobacter Pylori-Induced Gastric Pathology. Cancers 2019, 11, 372. [Google Scholar] [CrossRef]
- Simon, E.; Petke, D.; Boger, C.; Behrens, H.M.; Warneke, V.; Ebert, M.; Rocken, C. The spatial distribution of LGR5+ cells correlates with gastric cancer progression. PLoS ONE 2012, 7, e35486. [Google Scholar] [CrossRef]
- Zheng, Z.X.; Sun, Y.; Bu, Z.D.; Zhang, L.H.; Li, Z.Y.; Wu, A.W.; Wu, X.J.; Wang, X.H.; Cheng, X.J.; Xing, X.F.; et al. Intestinal stem cell marker LGR5 expression during gastric carcinogenesis. World J. Gastroenterol. 2013, 19, 8714–8721. [Google Scholar] [CrossRef]
- Oshima, H.; Matsunaga, A.; Fujimura, T.; Tsukamoto, T.; Taketo, M.M.; Oshima, M. Carcinogenesis in mouse stomach by simultaneous activation of the wnt signaling and prostaglandin e2 pathway. Gastroenterology 2006, 131, 1086–1095. [Google Scholar] [CrossRef]
- Hanaki, H.; Yamamoto, H.; Sakane, H.; Matsumoto, S.; Ohdan, H.; Sato, A.; Kikuchi, A. An anti-wnt5a antibody suppresses metastasis of gastric cancer cells in vivo by inhibiting receptor-mediated endocytosis. Mol. Cancer Ther. 2012, 11, 298–307. [Google Scholar] [CrossRef]
- Flanagan, D.J.; Barker, N.; Costanzo, N.S.D.; Mason, E.A.; Gurney, A.; Meniel, V.S.; Koushyar, S.; Austin, C.R.; Ernst, M.; Pearson, H.B.; et al. Frizzled-7 is required for wnt signaling in gastric tumors with and without apc mutations. Cancer Res. 2019, 79, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Qiu, X.; Xiao, J.; Wang, Q.; Wang, Y.; Zhang, Y.; Bai, D. The prognostic role of Leucine-rich repeat-containing G-protein-coupled receptor 5 in gastric cancer: A systematic review with meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 246–253. [Google Scholar] [CrossRef]
- Bu, Z.; Zheng, Z.; Zhang, L.; Li, Z.; Sun, Y.; Dong, B.; Wu, A.; Wu, X.; Wang, X.; Cheng, X.; et al. LGR5 is a promising biomarker for patients with stage I and II gastric cancer. Chin. J. Cancer Res. 2013, 25, 79–89. [Google Scholar] [PubMed]
- Bendell, J.C.; Murphy, J.E.; Mahalingam, D.; Halmos, B.; Sirard, C.A.; Landau, S.B.; Ryan, D.P. A Phase 1 study of DKN-01, an anti-DKK1 antibody, in combination with paclitaxel (pac) in patients with DKK1 relapsed or refractory esophageal cancer (EC) or gastro-esophageal junction tumors (GEJ). J. Clin. Oncol. 2016, 34, 111. [Google Scholar] [CrossRef]
- Kahn, M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014, 13, 513–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anti-Frizzled, Vantictumab (OMP-18R5). Available online: https://bciq.biocentury.com/products/anti-frizzled_vantictumab_(omp-18r5) (accessed on 21 April 2019).
- Seshagiri, S.; Stawiski, E.W.; Durinck, S.; Modrusan, Z.; Storm, E.E.; Conboy, C.B.; Chaudhuri, S.; Guan, Y.; Janakiraman, V.; Jaiswal, B.S.; et al. Recurrent R-spondin fusions in colon cancer. Nature 2012, 488, 660–664. [Google Scholar] [CrossRef]
- Chen, B.; Dodge, M.E.; Tang, W.; Lu, J.; Ma, Z.; Fan, C.W.; Wei, S.; Hao, W.; Kilgore, J.; Williams, N.S.; et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009, 5, 100–107. [Google Scholar] [CrossRef] [PubMed]
- The Wnt Homepage. Available online: https://web.stanford.edu/group/nusselab/cgi-bin/wnt/porcupine (accessed on 21 April 2019).
- Hazama, S.; Nakamura, Y.; Takenouchi, H.; Suzuki, N.; Tsunedomi, R.; Inoue, Y.; Tokuhisa, Y.; Iizuka, N.; Yoshino, S.; Takeda, K.; et al. A phase I study of combination vaccine treatment of five therapeutic epitope-peptides for metastatic colorectal cancer; safety, immunological response, and clinical outcome. J. Transl. Med. 2014, 12, 63. [Google Scholar] [CrossRef]


| Upregulated Wnt Pathway Promoting Genes | ||
| Wnt1 | Enhanced staining pattern in 98/180 of GC samples | [45] |
| normal gastric mucosa < precancerous lesion < early gastric adenocarcinoma < advanced gastric adenocarcinoma | [46] | |
| Wnt2B | In 2/8 GC samples | [47] |
| Wnt5A | Upregulated in 30% of GC | [48,49] |
| Wnt6 | WNT6 expression associated with tumor stage and nodal status | [50] |
| Wnt10A | In 3/6 GC samples | [51] |
| beta-catenin | Upregulated in GC compared to tumor-free tissue (p = 0.0046) | [52] |
| Loss of Function Mutations in Wnt Pathway Inhibitors | ||
| APC | In 7% of GC | [12] |
| In 15–18% of GC | [53,54] | |
| In 30–34% of GC | [52,55] | |
| Axin 1, Axin2 | 4/70 GC | [56] |
| RNF43 | 42/93 GC | [25] |
| In 33% of hypermutated GC | [12] | |
| In gastric cancer cell lines | [57] | |
| in 35.2% of early gastric cancer adenomas | [54] | |
| Epigenetic Modifications | ||
| APC | 37.7% in healthy tissues vs. 52.9% in GC | [58] |
| Dkk3 | 20/94 GC | [59] |
| 117/173 GC | [60] | |
| SFRP1 | 44% of GC | [61] |
| Regulation via microRNA | ||
| Upregulation | 41/352 microRNAs: miRNA-135 (APC) | [62,63] |
| Downregulated | 28/352 microRNAs: miRNA-103 (Axin2) | [62,64] |
| Single Nucleotide Polymorphisms (SNPs) | ||
| CTNNB1 | [65] | |
| Axin1 | 5 SNPs in 70 GC samples | [56] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, A.-S.; Sigal, M. The Role of Wnt and R-spondin in the Stomach During Health and Disease. Biomedicines 2019, 7, 44. https://doi.org/10.3390/biomedicines7020044
Fischer A-S, Sigal M. The Role of Wnt and R-spondin in the Stomach During Health and Disease. Biomedicines. 2019; 7(2):44. https://doi.org/10.3390/biomedicines7020044
Chicago/Turabian StyleFischer, Anne-Sophie, and Michael Sigal. 2019. "The Role of Wnt and R-spondin in the Stomach During Health and Disease" Biomedicines 7, no. 2: 44. https://doi.org/10.3390/biomedicines7020044