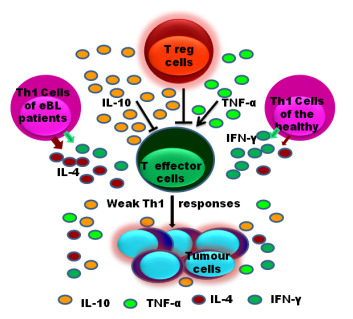
High Levels of IL-10 and CD4+CD25hi+ Treg Cells in Endemic Burkitt’s Lymphoma Patients
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Population and Inclusion Criteria
| Characteristic | Participants | Significance | ||||
|---|---|---|---|---|---|---|
| Malaria Patients (MAL) | eBL Patients | Controls (CON) | CON vs. MAL | CON vs. eBL | eBL vs. MAL | |
| Mean (95% CI) | p-Value | |||||
| Male # | 14 | 14 | 13 | - | - | - |
| Female # | 12 | 7 | 6 | - | - | - |
| Mean Age (range, years) | 8.6 (3 to 14) | 6.9 (3 to 11) | 8.7 (5 to 14) | - | - | - |
| WBC # | 8.75 | 8.17 | 5.95 | 0.0039 | 0.0017 | 0.5518 |
| (×103/μL) | (7.36 to 10.15) | (6.96 to 9.38) | (5.43 to 6.47) | |||
| HGB (g/dL) | 11.63 | 10.78 | 12.10 | - | 0.2906 * | - |
| (10.9 to 12.3) | (7.7 to 13.9) | (11.5 to 12.7) | ||||
| Parasitemia (parasites/μL) | 475 | - | - | - | - | - |
| (76 to 9275) | ||||||
2.2. Sample Collection and Processing
2.3. Parasitological and Haematological Examinations
2.4. Cell Phenotyping
2.5. Intracellular Cytokine Detection
2.6. In Vitro Stimulation
2.7. Flow Cytometric Acquisition and Analyses
2.8. Measurement of Cytokines by ELISA
2.9. Statistical Analyses
3. Results
3.1. Characteristics of Study Participants
3.2. Decrease in Responses by T Cells of eBL Patients

3.3. Th1/Th2 Responses to EBNA-1 in eBL Patients

3.4. High Plasma Level of TNF-α and IL-10 in eBL Patients

3.5. High Frequency of CD4+CD25hi+ Treg Cells in Patients

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mwanda, O.W.; Rochford, R.; Moormann, A.M.; Macneil, A.; Whalen, C.; Wilson, M.L. Burkitt’s lymphoma in Kenya: Geographical, age, gender and ethnic distribution. East Afr. Med. J. 2004, 8, S68–S77. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.A. Is Burkitt’s lymphoma sexy enough? Lancet 2006, 368, 2251–2252. [Google Scholar] [CrossRef]
- Epstein, M.A.; Achong, B.G.; Barr, Y.M. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet 1964, 1, 702–703. [Google Scholar] [CrossRef]
- Oyama, T.; Yamamoto, K.; Asano, N.; Oshiro, A.; Suzuki, R.; Kagami, Y.; Morishima, Y.; Takeuchi, K.; Izumo, T.; Mori, S.; et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: A study of 96 patients. Clin. Cancer Res. 2007, 13, 5124–5132. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, Y.; Yamamoto, K.; Asano, N.; Oyama, T.; Kinoshita, T.; Nakamura, S. Age-related Epstein-Barr virus-associated B-cell lymphoproliferative disorders: special references to lymphomas surrounding this newly recognized clinicopathologic disease. Cancer Sci. 2008, 99, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Piriou, E.; van Dort, K.; Nanlohy, N.M.; van Oers, M.H.; Miedema, F.; van Baarle, D. Loss of EBNA1-specific memory CD4+ and CD8+ T cells in HIV-infected patients progressing to AIDS-related non-Hodgkin lymphoma. Blood 2005, 106, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Fogg, M.H.; Wirth, L.J.; Posner, M.; Wang, F. Decreased EBNA-1-specific CD8+ T cells in patients with Epstein-Barr virus-associated nasopharyngeal carcinoma. Proc. Natl. Acad. Sci. USA 2009, 106, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Moormann, A.M.; Heller, K.N.; Chelimo, K.; Embury, P.; Ploutz-Snyder, R.; Otieno, J.A.; Oduor, M.; Munz, C.; Rochford, R. Children with endemic Burkitt lymphoma are deficient in EBNA1-specific IFN-gamma T cell responses. Int. J. Cancer 2009, 124, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zou, J.-Z.; di Renzo, L.; Wingberg, G.; Hu, L.-F.; Klein, E. A subpopulation of normal B cells latently infected with Epstein-Bar virus resembles Burkitt lymphoma cells in expressing EBNA-1 But not EBNA-2 or LMPl. J. Virol. 1995, 69, 3752–3758. [Google Scholar] [PubMed]
- Magrath, I.; Jain, V.; Bhatia, K. Epstein-Barr virus and Burkitt’s lymphoma. Semin. Cancer Biol. 1992, 3, 285–295. [Google Scholar] [PubMed]
- Magrath, I. The pathogenesis of Burkitt’s lymphoma. Adv. Cancer Res. 1990, 55, 133–270. [Google Scholar] [PubMed]
- Brady, G.; MacArthur, G.J.; Farrel, P.J. Epstein-Barr virus and Burkitt lymphoma. J. Clin. Path. 2007, 12, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Blake, N.; Lee, S.; Redchenko, I.; Thomas, W.; Steven, N.; Leese, A.; Steigerwald-Mullen, P.; Kurilla, M.G.; Frappier, L.; Rickinson, A. Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity 1997, 7, 791–802. [Google Scholar] [CrossRef]
- Voo, K.S.; Fu, T.; Wang, H.Y.; Tellam, J.; Heslop, H.E.; Brenner, M.K.; Rooney, C.M.; Wang, R.F. Evidence for the presentation of major histocompatibility complex class I-restricted Epstein-Barr virus nuclear antigen 1 peptides to CD8+ T lymphocytes. J. Exp. Med. 2004, 199, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Munz, C.; Bickham, K.L.; Subklewe, M.; Tsang, M.L.; Chahroudi, A.; Kurilla, M.G.; Zhang, D.; O’Donnell, M.; Steinman, R.M. Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J. Exp. Med. 2000, 191, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Nikiforow, S.; Bottomly, K.; Miller, G. CD4+ T-cell effectors inhibit Epstein-Barr virus-induced B-cell proliferation. J. Virol. 2001, 75, 3740–3752. [Google Scholar] [CrossRef] [PubMed]
- Paludan, C.; Bickham, K.; Nikiforow, S.; Tsang, M.L.; Goodman, K.; Hanekom, W.A.; Fonteneau, J.F.; Stevanovic, S.; Munz, C. Epstein-Barr nuclear antigen 1-specific CD4(+) Th1 cells kill Burkitt’s lymphoma cells. J. Immunol. 2002, 169, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Nikiforow, S.; Bottomly, K.; Miller, G.; Munz, C. Cytolytic CD4(+)-T-cell clones reactive to EBNA1 inhibit Epstein-Barr virus-induced B-cell proliferation. J. Virol. 2003, 77, 12088–12104. [Google Scholar] [CrossRef] [PubMed]
- Moormann, A.M.; Chelimo, K.; Sumba, P.O.; Tisch, D.J.; Rochford, R.; Lutzke, M.L.; Ploutz-Snyder, R.; Newton, D.; Kazura, J. Exposure to holoendemic malaria results in suppression of Epstein-Barr virus specific T cell immunosurveillance in Kenyan children. J. Infect. Dis. 2007, 195, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Lubega, J. T-helper 1 versus T-helper 2 lymphocyte immunodysregulation is the central factor in genesis of Burkitt lymphoma: Hypothesis. Infect. Agent. Cancer 2007, 2. [Google Scholar] [CrossRef] [PubMed]
- Pouniotis, D.S.; Proudfoot, O.; Bogdanoska, V.; Scalzo, K.; Kovacevic, S.; Coppel, R.L.; Plebanski, M. Selectively impaired CD8+ but not CD4+ T cell cycle arrest during priming as a consequence of dendritic cell interaction with plasmodium-infected red cells. J. Immunol. 2005, 175, 3525–3533. [Google Scholar] [CrossRef] [PubMed]
- Muller-Hermelink, N.; Braumuller, H.; Pichler, B.; Wieder, T.; Mailhammer, R.; Schaak, K.; Ghoreschi, K.; Yazdi, A.; Haubner, R.; Sander, C.A.; et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell 2008, 13, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Braumuller, H.; Wieder, T.; Brenner, E.; Assmann, S.; Hahn, M.; Alkhaled, M.; Schilbach, K.; Essmann, F.; Kneilling, M.; Griessinger, C.; et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013, 494, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Noyan, F.; Lee, Y.-S.; Hardtke-Wolenski, M.; Knoefel, A.-K.; Taubert, R.; Baron, U.; Manns, M.P.; Jaeckel, E. Donor-specific regulatory T cells generated on Donor B Cells are superior to CD4+CD25high cells in controlling alloimmune responses in humanized mice. Transplant Proc. 2013, 45, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Z.; Novak, A.J.; Ziesmer, S.C.; Witzig, T.E.; Ansell, S.M. Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin’s lymphoma. Cancer Res. 2006, 66, 10145–10152. [Google Scholar] [CrossRef] [PubMed]
- Strauss, L.; Bergmann, C.; Gooding, W.; Johnson, J.T.; Whiteside, T.L. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2007, 13, 6301–6311. [Google Scholar] [CrossRef] [PubMed]
- Roncarolo, M.G.; Gregori, S. Is FOXP3 a bona fide marker for human regulatory T cells? Eur. J. Immunol. 2008, 38, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, D.T.; Akbari, O.; Dekruyff, R.H. Regulatory T cells control the development of allergic disease and asthma. J. Allergy Clin. Immunol. 2003, 112, 480–487. [Google Scholar] [CrossRef]
- Roncarolo, M.G.; Bacchetta, R.; Bordignon, C.; Narula, S.; Levings, M.K. Type 1 T regulatory cells. Immunol. Rev. 2001, 182, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Burdin, N.; Peronne, C.; Banchereau, J.; Rousset, F. Epstein-Bar virus transformation induces B-Iymphocytes to produce human interleukin 10. J. Exp. Med. 1993, 177, 295–304. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Futagbi, G.; Gyan, B.; Nunoo, H.; Tetteh, J.K.A.; Welbeck, J.E.; Renner, L.A.; Ofori, M.; Dodoo, D.; Edoh, D.A.; Akanmori, B.D. High Levels of IL-10 and CD4+CD25hi+ Treg Cells in Endemic Burkitt’s Lymphoma Patients. Biomedicines 2015, 3, 224-236. https://doi.org/10.3390/biomedicines3030224
Futagbi G, Gyan B, Nunoo H, Tetteh JKA, Welbeck JE, Renner LA, Ofori M, Dodoo D, Edoh DA, Akanmori BD. High Levels of IL-10 and CD4+CD25hi+ Treg Cells in Endemic Burkitt’s Lymphoma Patients. Biomedicines. 2015; 3(3):224-236. https://doi.org/10.3390/biomedicines3030224
Chicago/Turabian StyleFutagbi, Godfred, Ben Gyan, Harriet Nunoo, John K.A. Tetteh, Jennifer E. Welbeck, Lorna Awo Renner, Michael Ofori, Daniel Dodoo, Dominic A. Edoh, and Bartholomew D. Akanmori. 2015. "High Levels of IL-10 and CD4+CD25hi+ Treg Cells in Endemic Burkitt’s Lymphoma Patients" Biomedicines 3, no. 3: 224-236. https://doi.org/10.3390/biomedicines3030224