Processing Adipose Tissue Samples in a GMP Environment Standardizes the Use of SVF in Cell Therapy Treatments: Data on 302 Patients
Abstract
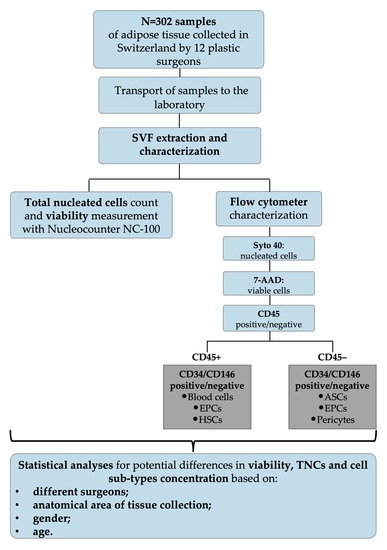
:1. Introduction
2. Materials and Methods
2.1. Patients’ Information
2.2. Adipose Tissue Sample Transportation
2.3. Adipose Tissue Sampling
2.4. GMP Isolation of SVF
2.4.1. SVF Characterization
2.4.2. Gating Strategy
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trujillo, M.E.; Scherer, P.E. Adipose Tissue-Derived Factors: Impact on Health and Disease. Endocr. Rev. 2006, 27, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.I.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Corvera, S. Cellular Heterogeneity in Adipose Tissues. Annu. Rev. Physiol. 2021, 83, 257–278. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Owen, M.; Friedenstein, A.J. Stromal Stem Cells: Marrow-Derived Osteogenic Precursors. In Ciba Foundation Symposium 136; John Wiley & Sons, Ltd.: Chichester, UK, 1988; Volume 136, pp. 42–60. [Google Scholar]
- Friedenstein, A.J.; I Piatetzky-Shapiro, I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. Development 1966, 16, 381–390. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef]
- Tremp, M.; Salemi, S.; Gobet, R.; Sulser, T.; Eberli, D. Adipose-Derived Stem Cells (ASCs) for Tissue Engineering. In Regenerative Medicine and Tissue Engineering; Eberli, D., Ed.; IntechOpen: Rijeka, Croatia, 2011; Volume 7. [Google Scholar] [CrossRef]
- Schäffler, A.; Büchler, C. Concise Review: Adipose Tissue-Derived Stromal Cells—Basic and Clinical Implications for Novel Cell-Based Therapies. Stem Cells 2007, 25, 818–827. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Artiles, M.; Bunnell, B.A. Adipose Stem Cells in Regenerative Medicine: Looking Forward. Front. Bioeng. Biotechnol. 2022, 9, 837464. [Google Scholar] [CrossRef]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef]
- Raposio, E.; Ciliberti, R. Clinical use of adipose-derived stem cells: European legislative issues. Ann. Med. Surg. 2017, 24, 61–64. [Google Scholar] [CrossRef]
- Raposio, E.; Bertozzi, N. Isolation of Ready-to-Use Adipose-Derived Stem Cell (ASC) Pellet for Clinical Applications and a Comparative Overview of Alternate Methods for ASC Isolation. Curr. Protoc. Stem Cell Biol. 2017, 41, 1F.17.1–1F.17.12. [Google Scholar] [CrossRef] [PubMed]
- Boada-Pladellorens, A.; Avellanet, M.; Pages-Bolibar, E.; Veiga, A. Stromal vascular fraction therapy for knee osteoarthritis: A systematic review. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221117879. [Google Scholar] [CrossRef] [PubMed]
- François, P.; Rusconi, G.; Arnaud, L.; Mariotta, L.; Giraudo, L.; Minonzio, G.; Veran, J.; Bertrand, B.; Dumoulin, C.; Grimaud, F.; et al. Inter-center comparison of good manufacturing practices-compliant stromal vascular fraction and proposal for release acceptance criteria: A review of 364 productions. Stem Cell Res. Ther. 2021, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Tallone, T.; Realini, C.; Böhmler, A.; Kornfeld, C.; Vassalli, G.; Moccetti, T.; Bardelli, S.; Soldati, G. Adult Human Adipose Tissue Contains Several Types of Multipotent Cells. J. Cardiovasc. Transl. Res. 2011, 4, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Astori, G.; Vignati, F.; Bardelli, S.; Tubio, M.; Gola, M.; Albertini, V.; Bambi, F.; Scali, G.; Castelli, D.; Rasini, V.; et al. “In vitro” and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J. Transl. Med. 2007, 5, 55. [Google Scholar] [CrossRef]
- Schmid, I.; Krall, W.J.; Uittenbogaart, C.H.; Braun, J.; Giorgi, J.V. Dead cell discrimination with 7-amino-actinomcin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry 1992, 13, 204–208. [Google Scholar] [CrossRef]
- Humpe, A.; Beck, C.; Schoch, R.; Kneba, M.; Horst, H.-A. Establishment and optimization of a flow cytometric method for evaluation of viability of CD34+ cells after cryopreservation and comparison with trypan blue exclusion staining. Transfusion 2005, 45, 1208–1213. [Google Scholar] [CrossRef]
- Minonzio, G.; Corazza, M.; Mariotta, L.; Gola, M.; Zanzi, M.; Gandolfi, E.; De Fazio, D.; Soldati, G. Frozen adipose-derived mesenchymal stem cells maintain high capability to grow and differentiate. Cryobiology 2014, 69, 211–216. [Google Scholar] [CrossRef]
- Tsekouras, A.; Mantas, D.; Tsilimigras, D.I.; Moris, D.; Kontos, M.; Zografos, G.C. Comparison of the Viability and Yield of Adipose-Derived Stem Cells (ASCs) from Different Donor Areas. Vivo 2017, 31, 1229–1234. [Google Scholar] [CrossRef]
- Jurgens, W.J.F.M.; Oedayrajsingh-Varma, M.J.; Helder, M.N.; ZandiehDoulabi, B.; Schouten, T.E.; Kuik, D.J.; Ritt, M.J.P.F.; van Milligen, F.J. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: Implications for cell-based therapies. Cell Tissue Res. 2008, 332, 415–426. [Google Scholar] [CrossRef]
- Bianconi, E.; Casadei, R.; Frabetti, F.; Ventura, C.; Facchin, F.; Canaider, S. Sex-Specific Transcriptome Differences in Human Adipose Mesenchymal Stem Cells. Genes 2020, 11, 909. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.K.; Lee, M.-J.; Karastergiou, K. Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity 2015, 23, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Novotny, N.M.; Crisostomo, P.R.; Lahm, T.; Abarbanell, A.; Meldrum, D.R. Sex Steroids and Stem Cell Function. Mol. Med. 2008, 14, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Boulet, N.; Briot, A.; Galitzky, J.; Bouloumié, A. The Sexual Dimorphism of Human Adipose Depots. Biomedicines 2022, 10, 2615. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Fried, S.K. Cellular mechanisms driving sex differences in adipose tissue biology and body shape in humans and mouse models. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2017; pp. 29–51. [Google Scholar] [CrossRef]
- Herold, J.; Kalucka, J. Angiogenesis in Adipose Tissue: The Interplay Between Adipose and Endothelial Cells. Front. Physiol. 2021, 11, 624903. [Google Scholar] [CrossRef]
- Holnthoner, W.; Hohenegger, K.; Husa, A.-M.; Muehleder, S.; Meinl, A.; Peterbauer-Scherb, A.; Redl, H. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. J. Tissue Eng. Regen. Med. 2015, 9, 127–136. [Google Scholar] [CrossRef]
- Traktuev, D.O.; Prater, D.N.; Merfeld-Clauss, S.; Sanjeevaiah, A.R.; Saadatzadeh, M.R.; Murphy, M.; Johnstone, B.H.; Ingram, D.A.; March, K.L. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ. Res. 2009, 104, 1410–1420. [Google Scholar] [CrossRef]
- Iba, T.; Naito, H.; Shimizu, S.; Rahmawati, F.N.; Wakabayashi, T.; Takakura, N. Isolation of tissue-resident endothelial stem cells and their use in regenerative medicine. Inflamm. Regen. 2019, 39, 9. [Google Scholar] [CrossRef]
- Saito, N.; Shirado, T.; Funabashi-Eto, H.; Wu, Y.; Mori, M.; Asahi, R.; Yoshimura, K. Purification and characterization of human adipose-resident microvascular endothelial progenitor cells. Sci. Rep. 2022, 12, 1775. [Google Scholar] [CrossRef]
- Patel, J.; Seppanen, E.J.; Rodero, M.P.; Wong, H.Y.; Donovan, P.; Neufeld, Z.; Fisk, N.M.; Francois, M.; Khosrotehrani, K. Functional Definition of Progenitors Versus Mature Endothelial Cells Reveals Key SoxF-Dependent Differentiation Process. Circulation 2017, 135, 786–805. [Google Scholar] [CrossRef]
- Paganelli, A.; Benassi, L.; Rossi, E.; Magnoni, C. Extracellular matrix deposition by adipose-derived stem cells and fibroblasts: A comparative study. Arch. Dermatol. Res. 2020, 312, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Errede, M.; Mangieri, D.; Longo, G.; Girolamo, F.; de Trizio, I.; Vimercati, A.; Serio, G.; Frei, K.; Perris, R.; Virgintino, D. Tunneling nanotubes evoke pericyte/endothelial communication during normal and tumoral angiogenesis. Fluids Barriers CNS 2018, 15, 28. [Google Scholar] [CrossRef]
- Planat-Benard, V.; Silvestre, J.-S.; Cousin, B.; André, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of Human Adipose Lineage Cells Toward Endothelial Cells: Physiological and therapeutic perspectives. Circulation 2004, 109, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, M.O.; Santidrian, A.; Minev, I.; Toth, R.; Draganov, D.; Nguyen, D.; Lander, E.; Berman, M.; Minev, B.; Szalay, A.A. The ratio of ADSCs to HSC-progenitors in adipose tissue derived SVF may provide the key to predict the outcome of stem-cell therapy. Clin. Transl. Med. 2018, 7, e5. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Shousha, W.G.; Abdo, S.M.; Mohamed, I.K.; El-Badri, N. Human Adipose-Derived Pericytes: Biological Characterization and Reprogramming into Induced Pluripotent Stem Cells. Cell Physiol. Biochem. 2020, 54, 271–286. [Google Scholar] [CrossRef]
- Covas, D.T.; Panepucci, R.A.; Fontes, A.M.; Silva, W.A.; Orellana, M.D.; Freitas, M.C.; Neder, L.; Santos, A.R.; Peres, L.C.; Jamur, M.C.; et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp. Hematol. 2008, 36, 642–654. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Catar, R.; Schoon, J.; Qazi, T.H.; Sass, F.A.; Jacobi, D.; Blankenstein, A.; Reinke, S.; Krüger, D.; Streitz, M.; et al. Multi-Parameter Analysis of Biobanked Human Bone Marrow Stromal Cells Shows Little Influence for Donor Age and Mild Comorbidities on Phenotypic and Functional Properties. Front. Immunol. 2019, 10, 2474. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cremona, M.; Rusconi, G.; Ferrario, A.; Mariotta, L.; Gola, M.; Soldati, G. Processing Adipose Tissue Samples in a GMP Environment Standardizes the Use of SVF in Cell Therapy Treatments: Data on 302 Patients. Biomedicines 2023, 11, 2533. https://doi.org/10.3390/biomedicines11092533
Cremona M, Rusconi G, Ferrario A, Mariotta L, Gola M, Soldati G. Processing Adipose Tissue Samples in a GMP Environment Standardizes the Use of SVF in Cell Therapy Treatments: Data on 302 Patients. Biomedicines. 2023; 11(9):2533. https://doi.org/10.3390/biomedicines11092533
Chicago/Turabian StyleCremona, Martina, Giulio Rusconi, Alessandro Ferrario, Luca Mariotta, Mauro Gola, and Gianni Soldati. 2023. "Processing Adipose Tissue Samples in a GMP Environment Standardizes the Use of SVF in Cell Therapy Treatments: Data on 302 Patients" Biomedicines 11, no. 9: 2533. https://doi.org/10.3390/biomedicines11092533