Mesenchymal Stem Cells Derived from Human Periapical Cysts and Their Implications in Regenerative Medicine
Abstract
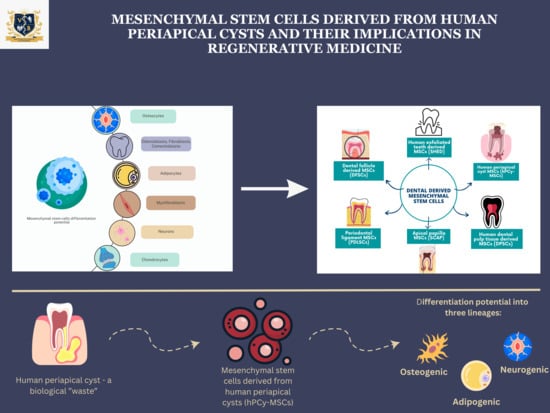
:1. Introduction
1.1. Biological Properties of Mesenchymal Stem Cells
1.2. Dental Mesenchymal Stem Cell Sources
2. Mesenchymal Stem Cells in Human Periapical Cysts
| Reference | Type of Study | Type of Samples | Identified Potential hPCY-MSCs | Differentiation Potential |
|---|---|---|---|---|
| [47] | In vitro | Periapical chronic granulation tissue | MSC-like cells | Osteogenic differentiation and production of calcified deposits |
| [24] | In vitro | Human periapical inflammatory tissue | MSC-like cells | Osteogenic differentiation potential |
| [48] | In vivo | Cell transplantation in mouse models | MSCs-like cells | Osteogenic differentiation and formation of mineralized tissue |
| [46] | In vitro | Human periapical cystic tissue | MSC-like cells | Osteogenic and adipogenic differentiation |
| [49] | In vitro | Human periapical cystic tissue | MSC-like cells | High osteogenic differentiation potential |
| [50] | In vitro | Human periapical cyst tissue | MSC-like cells | Neurogenic differentiation potential |
| [26] | In vitro | Human periapical lesions tissue | MSC-like cells | Osteogenic, adipogenic and chondrogenic differentiation potential |
| [51] | In vitro | Human periapical granuloma tissue | MSC-like cells | Immunomodulatory action and local healing |
| [54] | In vitro | Human periapical lesions | MSCs-like cells in inflammation infiltrate | Immunomodulatory response and pro-healing action associated with primary apical lesions |
| [55] | In vitro | Human periapical lesions | MSCs-like cells from the periapical lesions | Immunomodulatory action; a higher quantity of cells in acute lesions, suggesting their implication in the progress of the lesion |
| [58] | In vitro | Human periapical cysts | MSCs-like cells from periapical cysts | Osteogenic differentiation potential with an increased expression of the specific genes. |
| [64] | In vitro | Human periapical lesions | MSC-like cells | Osteogenic differentiation Immunomodulatory abilities Pro-angiogenetic activity |
3. Clinical Applications of hPCy-MSCs and Future Perspectives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lennon, D.P.; Caplan, A.I. Isolation of human marrow-derived mesenchymalstem cells. Exp. Hematol. 2006, 34, 1604–1605. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K.; Kern, S.; Klüter, H.; Eichler, H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004, 22, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Suchánek, J.; Soukup, T.; Ivancaková, R. Human dental pulp stem cells–isolation and long term cultivation. Acta Medica 2007, 50, 195–201. [Google Scholar]
- Winning, L.; El Karim, I.A.; Lundy, F.T. A Comparative Analysis of the Osteogenic Potential of Dental Mesenchymal Stem Cells. Stem Cells Dev. 2019, 28, 1050–1058. [Google Scholar] [CrossRef]
- Chang, J.Y.; Wang, C.; Jin, C. Self-renewal and multilineage differentiation of mouse dental epithelial stem cells. Stem Cell Res. 2013, 11, 990–1002. [Google Scholar] [CrossRef]
- Kobolak, J.; Dinnyes, A.; Memic, A.; Khademhosseini, A.; Mobasheri, A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 2016, 99, 62–68. [Google Scholar] [CrossRef]
- Peng, L.; Ye, L.; Zhou, X.D. Mesenchymal stem cells and tooth engineering. Int. J. Oral Sci. 2009, 1, 6–12. [Google Scholar] [CrossRef]
- Rochira, A.; Siculella, L.; Damiano, F. Concentrated Growth Factors (CGF) Induce Osteogenic Differentiation in Human Bone Marrow Stem Cells. Biology 2020, 9, 370. [Google Scholar] [CrossRef]
- Morsczeck, C.; Schmalz, G.; Reichert, T.E.; Völlner, F.; Galler, K.; Driemel, O. Somatic stem cells for regenerative dentistry. Clin. Oral Investig. 2008, 12, 113–118. [Google Scholar] [CrossRef]
- Palermo, A.; Giannotti, L.; Stanca, B.D.C.; Ferrante, F.; Gnoni, A.; Nitti, P.; Calabriso, N.; Demitri, C.; Damiano, F.; Batani, T.; et al. Use of CGF in Oral and Implant Surgery: From Laboratory Evidence to Clinical Evaluation. Int. J. Mol. Sci. 2022, 23, 15164. [Google Scholar] [CrossRef]
- Giannotti, L.; Stanca, B.D.C.; Nitti, P.; Spedicato, F.; Damiano, F.; Demitri, C.; Calabriso, N.; Carluccio, M.A.; Palermo, A.; Ferrante, F.; et al. Hydroxyapatite-Silicon Scaffold Promotes Osteogenic Differentiation of CGF Primary Cells. Biology 2023, 12, 528. [Google Scholar] [CrossRef]
- Rizk, A.; Rabie, A.B.M. Human dental pulp stem cells expressing transforming growth factor β3 transgene for cartilage-like tissue engineering. Cytotherapy 2013, 15, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.Z.; Govindasamy, V.; Musa, S.; Abu Kasim, N.H. Dental stem cells as an alternative source for cardiac regeneration. Med. Hypotheses 2013, 81, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Yamaguchi, S.; Amagasa, T. Multilineage Cells from Apical Pulp of Human Tooth with Immature Apex. Oral Sci. Int. 2007, 4, 45–58. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Iohara, K.; Sugiyama, M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 2009, 20, 435–440. [Google Scholar] [CrossRef]
- Morsczeck, C.; Götz, W.; Schierholz, J. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef]
- Kabir, R.; Gupta, M.; Aggarwal, A.; Sharma, D.; Sarin, A.; Kola, M.Z. Imperative role of dental pulp stem cells in regenerative therapies: A systematic review. Niger. J. Surg. 2014, 20, 1–8. [Google Scholar]
- Nosrat, I.V.; Smith, C.A.; Mullally, P.; Olson, L.; Nosrat, C.A. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur. J. Neurosci. 2004, 19, 2388–2398. [Google Scholar] [CrossRef]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef]
- Liao, J.; Al Shahrani, M.; Al-Habib, M.; Tanaka, T.; Huang, G.T. Cells isolated from inflamed periapical tissue express mesenchymal stem cell markers and are highly osteogenic. J. Endod. 2011, 37, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Kim, J.M.; Jung, I.H. Isolation and characterization of human periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed PDL tissue: In vitro and in vivo evaluations. J. Clin. Periodontol. 2011, 38, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Dokić, J.; Tomić, S.; Cerović, S.; Todorović, V.; Rudolf, R.; Colić, M. Characterization and immunosuppressive properties of mesenchymal stem cells from periapical lesions. J. Clin. Periodontol. 2012, 39, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Prockop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Konttinen, Y.T.; Kaivosoja, E.; Stegaev, V. Chapter 2: Extracellular matrix and tissue regeneration. In Regenerative Medicine: From Protocol to Patient; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2013; pp. 21–78. [Google Scholar]
- Mao, X.; Liu, Y.; Chen, C.; Shi, S. Mesenchymal Stem Cells and Their Role in Dental Medicine. Dent. Clin. N. Am. 2017, 61, 161–172. [Google Scholar] [CrossRef]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, F.J.; Bueno, C.; Insausti, C.L. Mesenchymal stem cells derived from dental tissues. Int. Endod. J. 2011, 44, 800–806. [Google Scholar] [CrossRef]
- Akiyama, K.; Chen, C.; Wang, D. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012, 10, 544–555. [Google Scholar] [CrossRef]
- Hargreaves, K.M.; Giesler, T.; Henry, M.; Wang, Y. Regeneration potential of the young permanent tooth: What does the future hold? J. Endod. 2008, 34 (Suppl. S7), S51–S56. [Google Scholar] [CrossRef] [PubMed]
- Leeb, C.; Jurga, M.; McGuckin, C.; Moriggl, R.; Kenner, L. Promising new sources for pluripotent stem cells. Stem Cell Rev. Rep. 2010, 6, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Kerkis, I.; Kerkis, A.; Dozortsev, D. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 2006, 184, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Marino, V.; Gronthos, S.; Bartold, P.M. Location of putative stem cells in human periodontal ligament. J. Periodontal Res. 2006, 41, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Yamaza, T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef]
- Morsczeck, C.; Völlner, F.; Saugspier, M.; Brandl, C.; Reichert, T.E.; Driemel, O.; Schmalz, G. Comparison of human dental follicle cells (DFCs) and stem cells from human exfoliated deciduous teeth (SHED) after neural differentiation in vitro. Clin. Oral Investig. 2010, 14, 433–440. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, S.; Liu, Y. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef] [PubMed]
- Kalra, K.; Tomar, P.C. Stem cell: Basics, classification and applications. Am. J. Phytomed. Clin. Ther. 2014, 2, 919–930. [Google Scholar]
- Garlet, G.P.; Horwat, R.; Ray, H.L., Jr. Expression analysis of wound healing genes in human periapical granulomas of progressive and stable nature. J. Endod. 2012, 38, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Paduano, F.; Tatullo, M. Cells isolated from human periapical cysts express mesenchymal stem cell-like properties. Int. J. Biol. Sci. 2013, 9, 1070–1078. [Google Scholar] [CrossRef]
- Maeda, H.; Wada, N.; Nakamuta, H.; Akamine, A. Human periapical granulation tissue contains osteogenic cells. Cell Tissue Res. 2004, 315, 203–208. [Google Scholar] [CrossRef]
- Patel, J.; Gudehithlu, K.P.; Dunea, G.; Arruda, J.A.; Singh, A.K. Foreign body-induced granulation tissue is a source of adult stem cells. Transl. Res. 2010, 155, 191–199. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Palmieri, F.; Tatullo, M. CD146 Expression Influences Periapical Cyst Mesenchymal Stem Cell Properties. Stem Cell Rev. Rep. 2016, 12, 592–603. [Google Scholar] [CrossRef]
- Marrelli, M.; Paduano, F.; Tatullo, M. Human periapical cyst-mesenchymal stem cells differentiate into neuronal cells. J. Dent. Res. 2015, 94, 843–852. [Google Scholar] [CrossRef]
- Araujo-Pires, A.C.; Biguetti, C.C.; Repeke, C.E. Mesenchymal stem cells as active prohealing and immunosuppressive agents in periapical environment: Evidence from human and experimental periapical lesions. J. Endod. 2014, 40, 1560–1565. [Google Scholar] [CrossRef]
- Chrepa, V.; Henry, M.A.; Daniel, B.J.; Diogenes, A. Delivery of Apical Mesenchymal Stem Cells into Root Canals of Mature Teeth. J. Dent. Res. 2015, 94, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Silva, B.S.F.; Silva, J.A.; Yamamoto-Silva, F.P.; Pinto-Júnior, D.D.; Gomez, R.S. Stem Cell Marker Expression in Persistent Apical Periodontitis. J. Endod. 2017, 43, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Carmo Souza, P.O.; Barbosa, M.G. Mesenchymal Stem Cell Marker Expression in Periapical Abscess. J. Endod. 2019, 45, 716–723. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Amantea, M. Adipose Tissue as a Strategic Source of Mesenchymal Stem Cells in Bone Regeneration: A Topical Review on the Most Promising Craniomaxillofacial Applications. Int. J. Mol. Sci. 2017, 18, 2140. [Google Scholar] [CrossRef]
- Xiong, J.; Mrozik, K.; Gronthos, S.; Bartold, P.M. Epithelial cell rests of Malassez contain unique stem cell populations capable of undergoing epithelial-mesenchymal transition. Stem Cells Dev. 2012, 21, 2012–2025. [Google Scholar] [CrossRef]
- Tatullo, M.; Falisi, G.; Amantea, M.; Rastelli, C.; Paduano, F.; Marrelli, M. Dental pulp stem cells and human periapical cyst mesenchymal stem cells in bone tissue regeneration: Comparison of basal and osteogenic differentiated gene expression of a newly discovered mesenchymal stem cell lineage. J. Biol. Regul. Homeost. Agents 2015, 29, 713–718. [Google Scholar]
- Tatullo, M.; Marrelli, M.; Paduano, F. The regenerative medicine in oral and maxillofacial surgery: The most important innovations in the clinical application of mesenchymal stem cells. Int. J. Med Sci. 2015, 12, 72–77. [Google Scholar] [CrossRef]
- Galler, K.M.; D’Souza, R.N. Tissue engineering approaches for regenerative dentistry. Regen. Med. 2011, 6, 111–124. [Google Scholar] [CrossRef]
- Graziano, A.; D’Aquino, R.; Angelis, M.G.C.-D.; De Francesco, F.; Giordano, A.; Laino, G.; Piattelli, A.; Traini, T.; De Rosa, A.; Papaccio, G. Scaffold’s surface geometry significantly affects human stem cell bone tissue engineering. J. Cell. Physiol. 2008, 214, 166–172. [Google Scholar] [CrossRef]
- Uccelli, A.; Laroni, A.; Freedman, M.S. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011, 10, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Dokić, J.; Tomić, S.; Marković, M.; Milosavljević, P.; Colić, M. Mesenchymal stem cells from periapical lesions modulate differentiation and functional properties of monocyte-derived dendritic cells. Eur. J. Immunol. 2013, 43, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mao, M.; Hu, N.; Wang, J.; Huang, J.; Gu, S. In vitro evaluation of periapical lesion-derived stem cells for dental pulp tissue engineering. FEBS Open Bio. 2022, 12, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Rao, M. Stem cells and regenerative medicine. Stem Cell Res. Ther. 2012, 3, 27. [Google Scholar] [CrossRef]
- Yang, J.W.; Shin, Y.Y.; Seo, Y.; Kim, H.S. Therapeutic Functions of Stem Cells from Oral Cavity: An Update. Int. J. Mol. Sci. 2020, 21, 4389. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yamato, M.; Kikuchi, A.; Okano, T.; Ishikawa, I. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng. 2005, 11, 469–478. [Google Scholar] [CrossRef]
- Feng, J.; Mantesso, A.; De Bari, C.; Nishiyama, A.; Sharpe, P.T. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc. Natl. Acad. Sci. USA 2011, 108, 6503–6508. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Hu, Y. Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent. Mater. 2019, 35, 1238–1253. [Google Scholar] [CrossRef]
- Gonmanee, T.; Sritanaudomchai, H.; Vongsavan, K. Neuronal differentiation of dental pulp stem cells from human permanent and deciduous teeth following coculture with rat auditory brainstem slices. Anat. Rec. 2020, 303, 2931–2946. [Google Scholar] [CrossRef]
- Zhang, W.; Walboomers, X.F.; Wolke, J.G.; Bian, Z.; Fan, M.W.; Jansen, J.A. Differentiation ability of rat postnatal dental pulp cells in vitro. Tissue Eng. 2005, 11, 357–368. [Google Scholar] [CrossRef]
- Yokoyama, T.; Mendoza, H.Y.; Tanaka, T. Regulation of CCl4-induced liver cirrhosis by hepatically differentiated human dental pulp stem cells. Hum Cell. 2019, 32, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, C.M.; An, S. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2014, 20, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M. Adipose tissue-derived therapeutics. Expert Opin. Biol. Ther. 2003, 3, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Codispoti, B.; Pacifici, A. Potential Use of Human Periapical Cyst-Mesenchymal Stem Cells (hPCy-MSCs) as a Novel Stem Cell Source for Regenerative Medicine Applications. Front. Cell Dev. Biol. 2017, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef]
- Wei, W.; An, Y.; An, Y.; Fei, D.; Wang, Q. Activation of autophagy in periodontal ligament mesenchymal stem cells promotes angiogenesis in periodontitis. J. Periodontol. 2018, 89, 718–727. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roi, A.; Roi, C.; Negruțiu, M.L.; Rusu, L.C.; Riviș, M. Mesenchymal Stem Cells Derived from Human Periapical Cysts and Their Implications in Regenerative Medicine. Biomedicines 2023, 11, 2436. https://doi.org/10.3390/biomedicines11092436
Roi A, Roi C, Negruțiu ML, Rusu LC, Riviș M. Mesenchymal Stem Cells Derived from Human Periapical Cysts and Their Implications in Regenerative Medicine. Biomedicines. 2023; 11(9):2436. https://doi.org/10.3390/biomedicines11092436
Chicago/Turabian StyleRoi, Alexandra, Ciprian Roi, Meda Lavinia Negruțiu, Laura Cristina Rusu, and Mircea Riviș. 2023. "Mesenchymal Stem Cells Derived from Human Periapical Cysts and Their Implications in Regenerative Medicine" Biomedicines 11, no. 9: 2436. https://doi.org/10.3390/biomedicines11092436