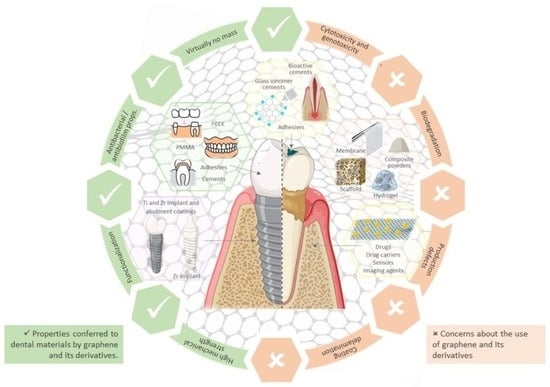
Can Graphene Pave the Way to Successful Periodontal and Dental Prosthetic Treatments? A Narrative Review
Abstract
:1. Introduction
2. Methodology
3. Graphene Derivatives
4. Antimicrobial Effects
5. Implant Surfaces and Osseointegration
5.1. Titanium Implants
5.2. Zirconia-Based Implants
| Material | Effects | Reference |
|---|---|---|
| Functionalized multiwalled carbon nanotubes on zirconia | Improved cell attachment | Kou et al., 2013 [118] |
| rGO/Dex | Osteogenic differentiation | Jung et al. 2015 [147] |
| GO | Osteogenic differentiation | Zhou et al. 2016 [107] |
| rGO/Ti | High hydrophilicity; rough surface; biocompatibility; enhanced ALP activity; collagen secretion; osteogenic differentiation | Qiu et al., 2017 [109] |
| GO/Ti/Dex | Promoted proliferation; accelerated osteogenic differentiation | Ren et al., 2017 [114] |
| nGO/PEG/PEI/siRNA | Osteogenic differentiation; osteointegration | Zhang et al., 2017 [148] |
| Single-layer graphene sheets | Osteogenic differentiation | Ming et al., 2018 [149] |
| GO/aspirin/Ti | Proliferation; osteogenic differentiation | Ren et al., 2018 [150] |
| GO/HA/chitosan | Promoted apatite formation | Karimi et al., 2019 [151] |
| Magnesium alloy with graphene nanoparticles | High cytocompatibility and osteogenic properties | Khan et al., 2019 [152] |
| GO/chitosan/HA | Osteogenic differentiation | Suo et al., 2019 [104] |
| GO/Ti | Biocompatibility; osteogenic differentiation | Di Carlo et al., 2020 [153] |
| GO | Re-osteogenesis | Qin et al., 2020 [73] |
| GO/Ti | Proliferation; adhesion, osteogenic differentiation, and osteointegration | Li and Wang, 2020 [110] |
| GO/Zirconia | Osteogenic differentiation | Desante et al., 2021 [20] |
| rGO nanosheets | Osteogenic differentiation | Lu et al., 2021 [154] |
| Graphene nanoplatelets and yttria-stabilized zirconia | Resistance to aging | Morales-Rodriguez et al., 2022 [130] |
| Reduced graphene oxide (rGO)-coated sandblasted | Accelerated healing rate with superior osseointegration | Shin et al., 2022 [155] |
6. Periodontal Tissue Regeneration
7. Restorative Materials
8. Prosthodontic Restorations
9. Concerns about the Use of Graphene and Its Derivatives
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Su, Y.; Ye, L.; Hu, C.; Zhang, Y.; Liu, J.; Shao, L. Periodontitis as a promoting factor of T2D: Current evidence and mechanisms. Int. J. Oral Sci. 2023, 15, 25. [Google Scholar] [CrossRef]
- Assery, N.M.; Jurado, C.A.; Assery, M.K.; Afrashtehfar, K.I. Peri-implantitis and systemic inflammation: A critical update. Saudi Dent. J. 2023, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Oczko, M.; Duś-Ilnicka, I.; Richards, P.; Thomsen, A.M.; Rasmussen, C. Rheumatoid arthritis patients’ oral health and disease activity. Int. J. Rheum. Dis. 2019, 22, 1538–1543. [Google Scholar] [CrossRef]
- Ancuta, C.; Chirieac, R.; Ancuta, E.; Tanculescu, O.; Solomon, S.M.; Fatu, A.M.; Doloca, A.; Iordache, C. Exploring the Role of Interleukin-6 Receptor Inhibitor Tocilizumab in Patients with Active Rheumatoid Arthritis and Periodontal Disease. J. Clin. Med. 2021, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- He, I.; Poirier, B.; Jensen, E.; Kaur, S.; Hedges, J.; Jesudason, S.; Jamieson, L.; Sethi, S. Demystifying the connection between periodontal disease and chronic kidney disease—An umbrella review. J. Periodontal Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiao, D.; Chen, R.; Zhu, F.; Gong, J.; Yan, F. The Association between Periodontitis and Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Biomed. Res. Int. 2021, 2021, 6692420. [Google Scholar] [CrossRef] [PubMed]
- Emami, E.; de Souza, R.F.; Kabawat, M.; Feine, J.S. The impact of edentulism on oral and general health. Int. J. Dent. 2013, 2013, 498305. [Google Scholar] [CrossRef]
- Rosenstiel, S.F.; Land, M.F.; Fujimoto, J. Contemporary Fixed Prosthodontics, 5th ed.; Elsevier: St. Louis, MO, USA, 2016. [Google Scholar]
- Shillingburg, H.T. Fundamentals of Fixed Prosthodontics, 3rd ed.; Quintessence Publishing Company, Ltd.: Chicago, IL, USA, 1997. [Google Scholar]
- Ciancaglini, R.; Gherlone, E.F.; Radaelli, G. Association between loss of occlusal support and symptoms of functional disturbances of the masticatory system. J. Oral Rehabil. 1999, 26, 248–253. [Google Scholar] [CrossRef]
- Jussila, P.; Kiviahde, H.; Näpänkangas, R.; Päkkilä, J.; Pesonen, P.; Sipilä, K.; Pirttiniemi, P.; Raustia, A. Prevalence of Temporomandibular Disorders in the Northern Finland Birth Cohort 1966. J. Oral Facial Pain Headache 2017, 31, 159–164. [Google Scholar] [CrossRef]
- Dawson, P.E. Functional Occlusion: From TMJ to Smile Design; Elsevier: Edinburgh, UK, 2006. [Google Scholar]
- Gennai, S.; Izzetti, R.; Pioli, M.C.; Music, L.; Graziani, F. Impact of rehabilitation versus edentulism on systemic health and quality of life in patients affected by periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2022, 49, 328–358. [Google Scholar] [CrossRef] [PubMed]
- Polzer, I.; Schimmel, M.; Müller, F.; Biffar, R. Edentulism as part of the general health problems of elderly adults. Int. Dent. J. 2010, 60, 143–155. [Google Scholar] [PubMed]
- Zafar, M.S.; Alnazzawi, A.A.; Alrahabi, M.; Fareed, M.A.; Najeeb, S.; Khurshid, Z. 18—Nanotechnology and nanomaterials in dentistry. In Advanced Dental Biomaterials; Khurshid, Z., Najeeb, S., Zafar, M.S., Sefat, F., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 477–505. [Google Scholar]
- Zheng, H.; Ji, Z.; Roy, K.R.; Gao, M.; Pan, Y.; Cai, X.; Wang, L.; Li, W.; Chang, C.H.; Kaweeteerawat, C.; et al. Engineered Graphene Oxide Nanocomposite Capable of Preventing the Evolution of Antimicrobial Resistance. ACS Nano 2019, 13, 11488–11499. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Mansukhani, N.D.; Guiney, L.M.; Ji, Z.; Zhao, Y.; Chang, C.H.; French, C.T.; Miller, J.F.; Hersam, M.C.; Nel, A.E.; et al. Identification and Optimization of Carbon Radicals on Hydrated Graphene Oxide for Ubiquitous Antibacterial Coatings. ACS Nano 2016, 10, 10966–10980. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [PubMed]
- Desante, G.; Labude, N.; Rütten, S.; Römer, S.; Kaufmann, R.; Zybała, R.; Jagiełło, J.; Lipińska, L.; Chlanda, A.; Telle, R.; et al. Graphene oxide nanofilm to functionalize bioinert high strength ceramics. Appl. Surf. Sci. 2021, 566, 150670. [Google Scholar] [CrossRef]
- Bullock, C.J.; Bussy, C. Biocompatibility Considerations in the Design of Graphene Biomedical Materials. Adv. Mater. Interfaces 2019, 6, 1900229. [Google Scholar] [CrossRef]
- Liu, J.H.; Yang, S.T.; Wang, H.; Chang, Y.; Cao, A.; Liu, Y. Effect of size and dose on the biodistribution of graphene oxide in mice. Nanomedicine 2012, 7, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- The Nobel Prize in Physics. Available online: https://www.nobelprize.org/prizes/physics/2010/summary/ (accessed on 13 March 2023).
- Yang, K.; Wang, J.; Chen, B. Facile fabrication of stable monolayer and few-layer graphene nanosheets as superior sorbents for persistent aromatic pollutant management in water. J. Mater. Chem. A 2014, 2, 18219–18224. [Google Scholar] [CrossRef]
- Radhi, A.; Mohamad, D.; Abdul Rahman, F.S.; Abdullah, A.M.; Hasan, H. Mechanism and factors influence of graphene-based nanomaterials antimicrobial activities and application in dentistry. J. Mater. Res. Technol. 2021, 11, 1290–1307. [Google Scholar] [CrossRef]
- Radovic, L.R. Probing the ‘elephant’: On the essential difference between graphenes and polycyclic aromatic hydrocarbons. Carbon 2021, 171, 798–805. [Google Scholar] [CrossRef]
- Li, X.; Liang, X.; Wang, Y.; Wang, D.; Teng, M.; Xu, H.; Zhao, B.; Han, L. Graphene-Based Nanomaterials for Dental Applications: Principles, Current Advances, and Future Outlook. Front. Bioeng. Biotechnol. 2022, 10, 804201. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Leal-Júnior, A.; Kumar, S. Multifunctional Integration of Optical Fibers and Nanomaterials for Aircraft Systems. Materials 2023, 16, 1433. [Google Scholar] [CrossRef] [PubMed]
- Ławkowska, K.; Pokrywczyńska, M.; Koper, K.; Kluth, L.A.; Drewa, T.; Adamowicz, J. Application of Graphene in Tissue Engineering of the Nervous System. Int. J. Mol. Sci. 2021, 23, 33. [Google Scholar] [CrossRef]
- Mamo, H.B.; Adamiak, M.; Kunwar, A. 3D printed biomedical devices and their applications: A review on state-of-the-art technologies, existing challenges, and future perspectives. J. Mech. Behav. Biomed. Mater. 2023, 143, 105930. [Google Scholar] [CrossRef]
- Silva, M.; Pinho, I.S.; Covas, J.A.; Alves, N.M.; Paiva, M.C. 3D printing of graphene-based polymeric nanocomposites for biomedical applications. Funct. Compos. Mater. 2021, 2, 8. [Google Scholar] [CrossRef]
- Kholghi Eshkalak, S.; Kowsari, E.; Ramakrishna, S. 17—3D printing of graphene-based composites and their applications in medicine and health care. In Innovations in Graphene-Based Polymer Composites; Rangappa, S.M., Parameswaranpillai, J., Ayyappan, V., Motappa, M.G., Siengchin, S., Soutis, C., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 463–485. [Google Scholar]
- Machado, M.; Oliveira, A.M.L.; Silva, G.A.; Bitoque, D.B.; Tavares Ferreira, J.; Pinto, L.A.; Ferreira, Q. Graphene Biosensors—A Molecular Approach. Nanomaterials 2022, 12, 1624. [Google Scholar]
- Sattari, S.; Adeli, M.; Beyranvand, S.; Nemati, M. Functionalized Graphene Platforms for Anticancer Drug Delivery. Int. J. Nanomed. 2021, 16, 5955–5980. [Google Scholar] [CrossRef]
- Ge, Z.; Yang, L.; Xiao, F.; Wu, Y.; Yu, T.; Chen, J.; Lin, J.; Zhang, Y. Graphene Family Nanomaterials: Properties and Potential Applications in Dentistry. Int. J. Biomater. 2018, 2018, 1539678. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Lei, L.; Zhang, H. Nanographene oxides carrying antisense walR RNA regulates the Enterococcus faecalis biofilm formation and its susceptibility to chlorhexidine. Lett. Appl. Microbiol. 2020, 71, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.C.; Brambilla, E.; Pires, P.M.; López-Castellano, A.; Alambiaga-Caravaca, A.M.; Lenardi, C.; Sauro, S. Physical-chemical and microbiological performances of graphene-doped PMMA for CAD/CAM applications before and after accelerated aging protocols. Dent. Mater. 2022, 38, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Agarwalla, S.V.; Ellepola, K.; Costa, M.C.F.d.; Fechine, G.J.M.; Morin, J.L.P.; Castro Neto, A.H.; Seneviratne, C.J.; Rosa, V. Hydrophobicity of graphene as a driving force for inhibiting biofilm formation of pathogenic bacteria and fungi. Dent. Mater. 2019, 35, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Abad-Coronel, C.; Calle, C.; Abril, G.; Paltán, C.A.; Fajardo, J.I. Fracture Resistance Analysis of CAD/CAM Interim Fixed Prosthodontic Materials: PMMA, Graphene, Acetal Resin and Polysulfone. Polymers 2023, 15, 1761. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene prehistory. Phys. Scr. 2012, 2012, 014003. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L.; Bangal, P.R. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 2010, 48, 1146–1152. [Google Scholar] [CrossRef]
- You, S.; Yu, J.; Sundqvist, B.; Talyzin, A.V. Solvation of graphite oxide in water–methanol binary polar solvents. Phys. Status Solidi 2012, 249, 2568–2571. [Google Scholar] [CrossRef]
- Xia, M.-Y.; Xie, Y.; Yu, C.-H.; Chen, G.-Y.; Li, Y.-H.; Zhang, T.; Peng, Q. Graphene-based nanomaterials: The promising active agents for antibiotics-independent antibacterial applications. J. Control. Release 2019, 307, 16–31. [Google Scholar] [CrossRef]
- Qi, X.; Jiang, F.; Zhou, M.; Zhang, W.; Jiang, X. Graphene oxide as a promising material in dentistry and tissue regeneration: A review. Smart Mater. Med. 2021, 2, 280–291. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.; Di Filippo, E.S.; Tumedei, M.; Marrone, M.; Fontana, A.; Ettorre, V.; Giordani, S.; Baldrighi, M.; Iezzi, G.; Piattelli, A.; et al. Human Dental Pulp Stem Cell Osteogenic Differentiation Seeded on Equine Bone Block with Graphene and Melatonin. Appl. Sci. 2021, 11, 3218. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Wu, M.-C.; Deokar, A.R.; Liao, J.-H.; Shih, P.-Y.; Ling, Y.-C. Graphene-Based Photothermal Agent for Rapid and Effective Killing of Bacteria. ACS Nano 2013, 7, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, F.; Oz, Y.; Quéniat, G.; Abderrahmani, A.; Foulon, C.; Lecoeur, M.; Sanyal, R.; Sanyal, A.; Boukherroub, R.; Szunerits, S. Photothermally triggered on-demand insulin release from reduced graphene oxide modified hydrogels. J. Control. Release 2017, 246, 164–173. [Google Scholar] [CrossRef]
- Cheng, X.; Wan, Q.; Pei, X. Graphene Family Materials in Bone Tissue Regeneration: Perspectives and Challenges. Nanoscale Res. Lett. 2018, 13, 289. [Google Scholar] [CrossRef]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-Based Nanomaterials for Tissue Engineering in the Dental Field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef]
- De Moraes, A.C.; Lima, B.A.; de Faria, A.F.; Brocchi, M.; Alves, O.L. Graphene oxide-silver nanocomposite as a promising biocidal agent against methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2015, 10, 6847–6861. [Google Scholar] [CrossRef]
- Zhu, Z.; Su, M.; Ma, L.; Ma, L.; Liu, D.; Wang, Z. Preparation of graphene oxide-silver nanoparticle nanohybrids with highly antibacterial capability. Talanta 2013, 117, 449–455. [Google Scholar] [CrossRef]
- Archana, S.; Kumar, K.Y.; Jayanna, B.K.; Olivera, S.; Anand, A.; Prashanth, M.K.; Muralidhara, H.B. Versatile Graphene oxide decorated by star shaped Zinc oxide nanocomposites with superior adsorption capacity and antimicrobial activity. J. Sci. Adv. Mater. Devices 2018, 3, 167–174. [Google Scholar] [CrossRef]
- Some, S.; Ho, S.-M.; Dua, P.; Hwang, E.; Shin, Y.H.; Yoo, H.; Kang, J.-S.; Lee, D.-k.; Lee, H. Dual Functions of Highly Potent Graphene Derivative–Poly-l-Lysine Composites To Inhibit Bacteria and Support Human Cells. ACS Nano 2012, 6, 7151–7161. [Google Scholar] [CrossRef] [PubMed]
- Mejías Carpio, I.E.; Santos, C.M.; Wei, X.; Rodrigues, D.F. Toxicity of a polymer-graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale 2012, 4, 4746–4756. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.J.; Compton, O.C.; An, Z.; Eryazici, I.; Nguyen, S.T. Successful Stabilization of Graphene Oxide in Electrolyte Solutions: Enhancement of Biofunctionalization and Cellular Uptake. ACS Nano 2012, 6, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-Structure-Dependent Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Pham, V.T.H.; Truong, V.K.; Quinn, M.D.J.; Notley, S.M.; Guo, Y.; Baulin, V.A.; Al Kobaisi, M.; Crawford, R.J.; Ivanova, E.P. Graphene Induces Formation of Pores That Kill Spherical and Rod-Shaped Bacteria. ACS Nano 2015, 9, 8458–8467. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Perreault, F.; de Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Jeyasubramanian, K.; Premanathan, M.; Subbiah, G.; Shin, H.S.; Kim, S.J. Graphene oxide nanopaint. Carbon 2014, 72, 328–337. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing Dental Pathogens Using Antibacterial Graphene Oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.U.; Jeong, S.; Seo, D.H.; Ahmed, R.; Lau, C.; Gali, N.K.; Ning, Z.; An, A.K. Mechanistic insight into the in vitro toxicity of graphene oxide against biofilm forming bacteria using laser-induced breakdown spectroscopy. Nanoscale 2018, 10, 4475–4487. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.M.; Lin, J.C.; Chen, Z.Y.; Wei, M.C.; Fu, Y.X.; Lu, S.S.; Yu, D.S.; Zhao, W. Enhanced antimicrobial activities of silver-nanoparticle-decorated reduced graphene nanocomposites against oral pathogens. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhang, L.; Shi, M.; Zhang, Y.; Wang, Q. Ti-GO-Ag nanocomposite: The effect of content level on the antimicrobial activity and cytotoxicity. Int. J. Nanomed. 2017, 12, 4209–4224. [Google Scholar] [CrossRef]
- Wei, J.; Qiao, S.; Zhang, X.; Li, Y.; Zhang, Y.; Wei, S.; Shi, J.; Lai, H. Graphene-Reinforced Titanium Enhances Soft Tissue Seal. Front. Bioeng. Biotechnol. 2021, 9, 665305. [Google Scholar] [CrossRef]
- Qin, W.; Wang, C.; Jiang, C.; Sun, J.; Yu, C.; Jiao, T. Graphene Oxide Enables the Reosteogenesis of Previously Contaminated Titanium In Vitro. J. Dent. Res. 2020, 99, 922–929. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, W.; Zhao, C.; Zhang, W.; Yan, Z. Graphene Coated Ti-6Al-4V Exhibits Antibacterial and Antifungal Properties Against Oral Pathogens. J. Prosthodont. 2022. [Google Scholar] [CrossRef]
- Guo, C.; Lu, R.; Wang, X.; Chen, S. Graphene Oxide-Modified Polyetheretherketone with Excellent Antibacterial Properties and Biocompatibility for Implant Abutment. Macromol. Res. 2021, 29, 351–359. [Google Scholar] [CrossRef]
- Yang, S.; Yu, W.; Zhang, J.; Han, X.; Wang, J.; Sun, D.; Shi, R.; Zhou, Y.; Zhang, H.; Zhao, J. The antibacterial property of zinc oxide/graphene oxide modified porous polyetheretherketone against S. sanguinis, F. nucleatum and P. gingivalis. Biomed. Mater. 2022, 17, 025013. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Etemad-Moghadam, S.; Alaeddini, M.; Miri Mousavi, R.s.; Bahador, A. DNA-aptamer-nanographene oxide as a targeted bio-theragnostic system in antimicrobial photodynamic therapy against Porphyromonas gingivalis. Sci. Rep. 2022, 12, 12161. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kang, K.; Luo, B.; Sun, X.; Lan, F.; He, J.; Wu, Y. Graphene oxide and mineralized collagen-functionalized dental implant abutment with effective soft tissue seal and romotely repeatable photodisinfection. Regen. Biomater. 2022, 9, rbac024. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, H.; Kanemoto, Y.; Hamamoto, A.; Shitomi, K.; Nishida, E.; Kato, A.; Sugaya, T.; Tanaka, S.; Aikawa, N.; Kawasaki, H.; et al. Sustained antibacterial coating with graphene oxide ultrathin film combined with cationic surface-active agents in a wet environment. Sci. Rep. 2022, 12, 16721. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Parker, S.; Chiniforush, N.; Bahador, A. Photoexcitation triggering via semiconductor Graphene Quantum Dots by photochemical doping with Curcumin versus perio-pathogens mixed biofilms. Photodiagnosis Photodyn. Ther. 2019, 28, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Trusek, A.; Kijak, E. Drug Carriers Based on Graphene Oxide and Hydrogel: Opportunities and Challenges in Infection Control Tested by Amoxicillin Release. Materials 2021, 14, 3182. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef]
- Vi, T.T.T.; Rajesh Kumar, S.; Rout, B.; Liu, C.H.; Wong, C.B.; Chang, C.W.; Chen, C.H.; Chen, D.W.; Lue, S.J. The Preparation of Graphene Oxide-Silver Nanocomposites: The Effect of Silver Loads on Gram-Positive and Gram-Negative Antibacterial Activities. Nanomaterials 2018, 8, 163. [Google Scholar] [CrossRef]
- Moghayedi, M.; Goharshadi, E.K.; Ghazvini, K.; Ahmadzadeh, H.; Ranjbaran, L.; Masoudi, R.; Ludwig, R. Kinetics and mechanism of antibacterial activity and cytotoxicity of Ag-RGO nanocomposite. Colloids Surf. B Biointerfaces 2017, 159, 366–374. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Zhang, C.; Zeng, G.M.; Gong, J.L.; Ou, X.M.; Huan, S.Y. Easily separated silver nanoparticle-decorated magnetic graphene oxide: Synthesis and high antibacterial activity. J. Colloid Interface Sci. 2016, 471, 94–102. [Google Scholar] [CrossRef]
- Ristic, B.Z.; Milenkovic, M.M.; Dakic, I.R.; Todorovic-Markovic, B.M.; Milosavljevic, M.S.; Budimir, M.D.; Paunovic, V.G.; Dramicanin, M.D.; Markovic, Z.M.; Trajkovic, V.S. Photodynamic antibacterial effect of graphene quantum dots. Biomaterials 2014, 35, 4428–4435. [Google Scholar] [CrossRef]
- Aunkor, M.T.H.; Raihan, T.; Prodhan, S.H.; Metselaar, H.S.C.; Malik, S.U.F.; Azad, A.K. Antibacterial activity of graphene oxide nanosheet against multidrug resistant superbugs isolated from infected patients. R. Soc. Open Sci. 2020, 7, 200640. [Google Scholar] [CrossRef]
- Jia, Z.; Shi, Y.; Xiong, P.; Zhou, W.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. From Solution to Biointerface: Graphene Self-Assemblies of Varying Lateral Sizes and Surface Properties for Biofilm Control and Osteodifferentiation. ACS Appl. Mater. Interfaces 2016, 8, 17151–17165. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene Oxide-Based Nanocomposites Decorated with Silver Nanoparticles as an Antibacterial Agent. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- De Faria, A.F.; Perreault, F.; Shaulsky, E.; Arias Chavez, L.H.; Elimelech, M. Antimicrobial Electrospun Biopolymer Nanofiber Mats Functionalized with Graphene Oxide-Silver Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 12751–12759. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhao, Q.; Lu, S.; Fu, Y.; Yu, D.; Zhao, W. Inhibitory effect of reduced graphene oxide-silver nanocomposite on progression of artificial enamel caries. J. Appl. Oral Sci. 2018, 27, e20180042. [Google Scholar] [CrossRef]
- Yu, C.H.; Chen, G.Y.; Xia, M.Y.; Xie, Y.; Chi, Y.Q.; He, Z.Y.; Zhang, C.L.; Zhang, T.; Chen, Q.M.; Peng, Q. Understanding the sheet size-antibacterial activity relationship of graphene oxide and the nano-bio interaction-based physical mechanisms. Colloids Surf. B Biointerfaces 2020, 191, 111009. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Zhang, H.; Lei, L. Nano-graphene oxide with antisense vicR RNA reduced exopolysaccharide synthesis and biofilm aggregation for Streptococcus mutans. Dent. Mater. J. 2020, 39, 278–286. [Google Scholar] [CrossRef]
- Bacali, C.; Baldea, I.; Moldovan, M.; Carpa, R.; Olteanu, D.E.; Filip, G.A.; Nastase, V.; Lascu, L.; Badea, M.; Constantiniuc, M.; et al. Flexural strength, biocompatibility, and antimicrobial activity of a polymethyl methacrylate denture resin enhanced with graphene and silver nanoparticles. Clin. Oral Investig. 2020, 24, 2713–2725. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.-R.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, S.; Cho, S.-P.; Hong, B.H.; Choung, P.-H.; Chung, T.D.; et al. Graphene-incorporated chitosan substrata for adhesion and differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2013, 1, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liu, Z. Graphene in biomedicine: Opportunities and challenges. Nanomedicine 2011, 6, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, Y.; Li, K.; Huang, L.; Huang, S.; Zhao, B.; Zheng, X. Microstructure and wear behavior of graphene nanosheets-reinforced zirconia coating. Ceram. Int. 2014, 40, 12821–12829. [Google Scholar] [CrossRef]
- Su, J.; Chen, Y.; Huang, Q. Graphene nanosheet-induced toughening of yttria-stabilized zirconia. Appl. Phys. A 2016, 123, 10. [Google Scholar] [CrossRef]
- Yi, Z.; Merenda, A.; Kong, L.; Radenovic, A.; Majumder, M.; Dumée, L.F. Single step synthesis of Schottky-like hybrid graphene—Titania interfaces for efficient photocatalysis. Sci. Rep. 2018, 8, 8154. [Google Scholar] [CrossRef]
- Rho, K.; Park, C.; Alam, K.; Kim, D.; Ji, M.-K.; Lim, H.-P.; Cho, H. Biological Effects of Plasma-Based Graphene Oxide Deposition on Titanium. J. Nanomater. 2019, 2019, 9124989. [Google Scholar] [CrossRef]
- Suo, L.; Jiang, N.; Wang, Y.; Wang, P.; Chen, J.; Pei, X.; Wang, J.; Wan, Q. The enhancement of osseointegration using a graphene oxide/chitosan/hydroxyapatite composite coating on titanium fabricated by electrophoretic deposition. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 635–645. [Google Scholar] [CrossRef]
- Dubey, N.; Morin, J.L.P.; Luong-Van, E.K.; Agarwalla, S.V.; Silikas, N.; Castro Neto, A.H.; Rosa, V. Osteogenic potential of graphene coated titanium is independent of transfer technique. Materialia 2020, 9, 100604. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Yu, X.; Fan, H.; Wang, Q.; Yan, S.; Wang, L.; Jiang, W. Graphene nanosheet/titanium carbide composites of a fine-grained structure and improved mechanical properties. Ceram. Int. 2016, 42, 165–172. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, P.; Li, X.; Liu, H.; Ge, S. Bioactivity of periodontal ligament stem cells on sodium titanate coated with graphene oxide. Sci. Rep. 2016, 6, 19343. [Google Scholar] [CrossRef]
- Qiu, J.; Geng, H.; Wang, D.; Qian, S.; Zhu, H.; Qiao, Y.; Qian, W.; Liu, X. Layer-Number Dependent Antibacterial and Osteogenic Behaviors of Graphene Oxide Electrophoretic Deposited on Titanium. ACS Appl. Mater. Interfaces 2017, 9, 12253–12263. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Guo, J.; Geng, H.; Qian, W.; Liu, X. Three-dimensional porous graphene nanosheets synthesized on the titanium surface for osteogenic differentiation of rat bone mesenchymal stem cells. Carbon 2017, 125, 227–235. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z. Involvement of FAK/P38 Signaling Pathways in Mediating the Enhanced Osteogenesis Induced by Nano-Graphene Oxide Modification on Titanium Implant Surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wu, K.; Wang, C.; Guo, Y.; Lu, R.; Wang, X.; Chen, S. Graphene Oxide Loaded on TiO2-Nanotube-Modified Ti Regulates the Behavior of Human Gingival Fibroblasts. Int. J. Mol. Sci. 2022, 23, 8723. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.E.; Choi, J.; Joung, Y.K.; Park, K.; Han, D.K. Controlled release of bone morphogenetic protein (BMP)-2 from nanocomplex incorporated on hydroxyapatite-formed titanium surface. J. Control. Release 2012, 160, 676–684. [Google Scholar] [CrossRef]
- La, W.G.; Jin, M.; Park, S.; Yoon, H.H.; Jeong, G.J.; Bhang, S.H.; Park, H.; Char, K.; Kim, B.S. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int. J. Nanomed. 2014, 9 (Suppl. 1), 107–116. [Google Scholar] [CrossRef]
- Ren, N.; Li, J.; Qiu, J.; Yan, M.; Liu, H.; Ji, D.; Huang, J.; Yu, J.; Liu, H. Growth and accelerated differentiation of mesenchymal stem cells on graphene-oxide-coated titanate with dexamethasone on surface of titanium implants. Dent. Mater. 2017, 33, 525–535. [Google Scholar] [CrossRef]
- Qian, W.; Qiu, J.; Liu, X. Minocycline hydrochloride-loaded graphene oxide films on implant abutments for peri-implantitis treatment in beagle dogs. J. Periodontol. 2020, 91, 792–799. [Google Scholar] [CrossRef]
- Özcan, M.; Volpato, C.A.M.; Hian, L.; Karahan, B.D.; Cesar, P.F. Graphene for Zirconia and Titanium Composites in Dental Implants: Significance and Predictions. Curr. Oral Health Rep. 2022, 9, 66–74. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C 2019, 98, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Kou, W.; Akasaka, T.; Watari, F.; Sjögren, G. An in vitro evaluation of the biological effects of carbon nanotube-coated dental zirconia. ISRN Dent. 2013, 2013, 296727. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Kim, H.S.; Alam, K.; Ji, M.K.; Cho, H.S.; Lim, H.P. Direct-Deposited Graphene Oxide on Dental Implants for Antimicrobial Activities and Osteogenesis. Int. J. Nanomed. 2021, 16, 5745–5754. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Khan, M.M.; Chee, H.A.; Wong, Y.; Ganesan, P.; Kutty, M.; Sutharsini, U.; Chew, W.K.; Niakan, A. Sintering behaviour and properties of graphene oxide-doped Y-TZP ceramics. Ceram. Int. 2016, 42, 17620–17625. [Google Scholar] [CrossRef]
- Smirnov, A.; Solís Pinargote, N.W.; Peretyagin, N.; Pristinskiy, Y.; Peretyagin, P.; Bartolomé, J.F. Zirconia Reduced Graphene Oxide Nano-Hybrid Structure Fabricated by the Hydrothermal Reaction Method. Materials 2020, 13, 687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, Z.; Zhao, L.; Liu, W.; Si, P.; Lan, J. Synthesis and characterization of multilayer graphene oxide on yttria-zirconia ceramics for dental implant. J. Mater. Res. 2020, 35, 2466–2477. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Z.; Zhao, L. Mechanical properties and tribological behaviors of yttria-zirconia ceramics with additions of graphene oxide by hot-press sintering for dental implants. Surf. Topogr. Metrol. Prop. 2020, 8, 035010. [Google Scholar] [CrossRef]
- Rincón, A.; Moreno, R.; Gutiérrez-González, C.F.; Sainz, R.; Salvador, M.D.; Borrell, A. Colloidal processing of fully stabilized zirconia laminates comprising graphene oxide-enriched layers. J. Eur. Ceram. Soc. 2016, 36, 1797–1804. [Google Scholar] [CrossRef]
- Shin, J.-H.; Hong, S.-H. Fabrication and properties of reduced graphene oxide reinforced yttria-stabilized zirconia composite ceramics. J. Eur. Ceram. Soc. 2014, 34, 1297–1302. [Google Scholar] [CrossRef]
- Obradović, N.; Kern, F. Properties of 3Y-TZP zirconia ceramics with graphene addition obtained by spark plasma sintering. Ceram. Int. 2018, 44, 16931–16936. [Google Scholar] [CrossRef]
- López-Pernía, C.; Muñoz-Ferreiro, C.; González-Orellana, C.; Morales-Rodríguez, A.; Gallardo-López, Á.; Poyato, R. Optimizing the homogenization technique for graphene nanoplatelet/yttria tetragonal zirconia composites: Influence on the microstructure and the electrical conductivity. J. Alloys Compd. 2018, 767, 994–1002. [Google Scholar] [CrossRef]
- Echeberria, J.; Ollo, J.; Bocanegra-Bernal, M.H.; Garcia-Reyes, A.; Domínguez-Rios, C.; Aguilar-Elguezabal, A.; Reyes-Rojas, A. Sinter and hot isostatic pressing (HIP) of multi-wall carbon nanotubes (MWCNTs) reinforced ZTA nanocomposite: Microstructure and fracture toughness. Int. J. Refract. Met. Hard Mater. 2010, 28, 399–406. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, Y.; Chen, W.; Li, X.; Zheng, Q.; Li, K.; Guo, R. Fabrication and properties of in situ reduced graphene oxide-toughened zirconia composite ceramics. J. Am. Ceram. Soc. 2018, 101, 3498–3507. [Google Scholar] [CrossRef]
- Morales-Rodríguez, A.; González-Orellana, C.; Pérez-García, A.A.; López-Pernía, C.; Muñoz-Ferreiro, C.; Poyato, R.; Gallardo-López, Á. Ageing-resistant zirconia/graphene-based nanostructures composites for use as biomaterials. J. Eur. Ceram. Soc. 2022, 42, 1784–1795. [Google Scholar] [CrossRef]
- Ando, Y. Carbon nanotube: The inside story. J. Nanosci. Nanotechnol. 2010, 10, 3726–3738. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Chen, H.; Wang, B.; Gao, D.; Guan, M.; Zheng, L.; Ouyang, H.; Chai, Z.; Zhao, Y.; Feng, W. Broad-Spectrum Antibacterial Activity of Carbon Nanotubes to Human Gut Bacteria. Small 2013, 9, 2735–2746. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and Faster “Nano Darts” Kill More Bacteria: A Study of Antibacterial Activity of Individually Dispersed Pristine Single-Walled Carbon Nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.F.; Elimelech, M. Toxic effects of single-walled carbon nanotubes in the development of E. coli biofilm. Environ. Sci. Technol. 2010, 44, 4583–4589. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial and anti-adhesive properties of carbon nanotube-based surfaces for medical applications: A systematic review. iScience 2021, 24, 102001. [Google Scholar] [CrossRef]
- Garmendia, N.; Grandjean, S.; Chevalier, J.; Diaz, L.A.; Torrecillas, R.; Obieta, I. Zirconia–multiwall carbon nanotubes dense nano-composites with an unusual balance between crack and ageing resistance. J. Eur. Ceram. Soc. 2011, 31, 1009–1014. [Google Scholar] [CrossRef]
- Gallardo-López, Á.; Muñoz-Ferreiro, C.; López-Pernía, C.; Jiménez-Piqué, E.; Gutiérrez-Mora, F.; Morales-Rodríguez, A.; Poyato, R. Critical Influence of the Processing Route on the Mechanical Properties of Zirconia Composites with Graphene Nanoplatelets. Materials 2020, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, F.; Inchingolo, F.; Greco Lucchina, A.; Scogna, G.; Scarano, A. Graphene-doped Poly(methyl-methacrylate) as an enhanced biopolymer for medical device and dental implant. J. Biol. Regul. Homeost. Agents 2021, 35, 195–204. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Malcangi, G.; Inchingolo, A.D.; Mancini, A.; Palmieri, G.; Di Pede, C.; Piras, F.; Inchingolo, F.; Dipalma, G.; Patano, A. Potential of Graphene-Functionalized Titanium Surfaces for Dental Implantology: Systematic Review. Coatings 2023, 13, 725. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, M.; Zhao, R.; Liu, H.; Li, K.; Tian, M.; Niu, L.; Xie, R.; Bai, S. Clinical applications of polyetheretherketone in removable dental prostheses: Accuracy, characteristics, and performance. Polymers 2022, 14, 4615. [Google Scholar] [CrossRef]
- Qin, W.; Li, Y.; Ma, J.; Liang, Q.; Cui, X.; Jia, H.; Tang, B. Osseointegration and biosafety of graphene oxide wrapped porous CF/PEEK composites as implantable materials: The role of surface structure and chemistry. Dent. Mater. 2020, 36, 1289–1302. [Google Scholar] [CrossRef]
- Jung, H.S.; Lee, T.; Kwon, I.K.; Kim, H.S.; Hahn, S.K.; Lee, C.S. Surface modification of multipass caliber-rolled Ti alloy with dexamethasone-loaded graphene for dental applications. ACS Appl. Mater. Interfaces 2015, 7, 9598–9607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Q.; Song, W.; Wu, K.; Zhang, Y.; Zhao, Y. Dual-Functionalized Graphene Oxide Based siRNA Delivery System for Implant Surface Biomodification with Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2017, 9, 34722–34735. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Lv, L.; Du, F.; Niu, T.; Chen, T.; Xia, D.; Wang, S.; Zhao, X.; Liu, J.; Liu, Y.; et al. Effects of thermal treatment on the adhesion strength and osteoinductive activity of single-layer graphene sheets on titanium substrates. Sci. Rep. 2018, 8, 8141. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Pan, S.; Li, H.; Li, Y.; He, L.; Zhang, S.; Che, J.; Niu, Y. Effects of aspirin-loaded graphene oxide coating of a titanium surface on proliferation and osteogenic differentiation of MC3T3-E1 cells. Sci. Rep. 2018, 8, 15143. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Kharaziha, M.; Raeissi, K. Electrophoretic deposition of chitosan reinforced graphene oxide-hydroxyapatite on the anodized titanium to improve biological and electrochemical characteristics. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Al-Khureif, A.A.; Saadaldin, S.A.; Mohamed, B.A.; Musaibah, A.S.; Divakar, D.D.; Eldwakhly, E. Graphene oxide-based experimental silane primers enhance shear bond strength between resin composite and zirconia. Eur. J. Oral Sci. 2019, 127, 570–576. [Google Scholar] [CrossRef]
- Di Carlo, R.; Di Crescenzo, A.; Pilato, S.; Ventrella, A.; Piattelli, A.; Recinella, L.; Chiavaroli, A.; Giordani, S.; Baldrighi, M.; Camisasca, A.; et al. Osteoblastic Differentiation on Graphene Oxide-Functionalized Titanium Surfaces: An In Vitro Study. Nanomaterials 2020, 10, 654. [Google Scholar] [CrossRef]
- Lu, J.; Sun, J.; Zou, D.; Song, J.; Yang, S. Graphene-Modified Titanium Surface Enhances Local Growth Factor Adsorption and Promotes Osteogenic Differentiation of Bone Marrow Stromal Cells. Front. Bioeng. Biotechnol. 2020, 8, 621788. [Google Scholar] [CrossRef]
- Shin, Y.C.; Bae, J.H.; Lee, J.H.; Raja, I.S.; Kang, M.S.; Kim, B.; Hong, S.W.; Huh, J.B.; Han, D.W. Enhanced osseointegration of dental implants with reduced graphene oxide coating. Biomater. Res. 2022, 26, 11. [Google Scholar] [CrossRef]
- Scantlebury, T.V. 1982-1992: A decade of technology development for guided tissue regeneration. J. Periodontol. 1993, 64, 1129–1137. [Google Scholar] [CrossRef]
- Radunovic, M.; De Colli, M.; De Marco, P.; Di Nisio, C.; Fontana, A.; Piattelli, A.; Cataldi, A.; Zara, S. Graphene oxide enrichment of collagen membranes improves DPSCs differentiation and controls inflammation occurrence. J. Biomed. Mater. Res. A 2017, 105, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- De Marco, P.; Zara, S.; De Colli, M.; Radunovic, M.; Lazović, V.; Ettorre, V.; Di Crescenzo, A.; Piattelli, A.; Cataldi, A.; Fontana, A. Graphene oxide improves the biocompatibility of collagen membranes in an in vitro model of human primary gingival fibroblasts. Biomed. Mater. 2017, 12, 055005. [Google Scholar] [CrossRef] [PubMed]
- Nishida, E.; Miyaji, H.; Kato, A.; Takita, H.; Iwanaga, T.; Momose, T.; Ogawa, K.; Murakami, S.; Sugaya, T.; Kawanami, M. Graphene oxide scaffold accelerates cellular proliferative response and alveolar bone healing of tooth extraction socket. Int. J. Nanomed. 2016, 11, 2265–2277. [Google Scholar] [CrossRef]
- Zhou, T.; Li, G.; Lin, S.; Tian, T.; Ma, Q.; Zhang, Q.; Shi, S.; Xue, C.; Ma, W.; Cai, X.; et al. Electrospun Poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/Graphene Oxide Scaffold: Enhanced Properties and Promoted in vivo Bone Repair in Rats. ACS Appl. Mater. Interfaces 2017, 9, 42589–42600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chang, Q.; Xu, L.; Li, G.; Yang, G.; Ding, X.; Wang, X.; Cui, D.; Jiang, X. Graphene Oxide-Copper Nanocomposite-Coated Porous CaP Scaffold for Vascularized Bone Regeneration via Activation of Hif-1α. Adv. Healthc. Mater. 2016, 5, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Whited, B.M.; Whitney, J.R.; Hofmann, M.C.; Xu, Y.; Rylander, M.N. Pre-osteoblast infiltration and differentiation in highly porous apatite-coated PLLA electrospun scaffolds. Biomaterials 2011, 32, 2294–2304. [Google Scholar] [CrossRef]
- Ben-David, D.; Srouji, S.; Shapira-Schweitzer, K.; Kossover, O.; Ivanir, E.; Kuhn, G.; Müller, R.; Seliktar, D.; Livne, E. Low dose BMP-2 treatment for bone repair using a PEGylated fibrinogen hydrogel matrix. Biomaterials 2013, 34, 2902–2910. [Google Scholar] [CrossRef]
- Vaquette, C.; Ivanovski, S.; Hamlet, S.M.; Hutmacher, D.W. Effect of culture conditions and calcium phosphate coating on ectopic bone formation. Biomaterials 2013, 34, 5538–5551. [Google Scholar] [CrossRef]
- Levengood, S.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Klébert, S.; Balázsi, C.; Balázsi, K.; Bódis, E.; Fazekas, P.; Keszler, A.M.; Szépvölgyi, J.; Károly, Z. Spark plasma sintering of graphene reinforced hydroxyapatite composites. Ceram. Int. 2015, 41, 3647–3652. [Google Scholar] [CrossRef]
- Raucci, M.G.; Giugliano, D.; Longo, A.; Zeppetelli, S.; Carotenuto, G.; Ambrosio, L. Comparative facile methods for preparing graphene oxide-hydroxyapatite for bone tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2204–2216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, J.; Li, H. Synthesis of hydroxyapatite-reduced graphite oxide nanocomposites for biomedical applications: Oriented nucleation and epitaxial growth of hydroxyapatite. J. Mater. Chem. B 2013, 1, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Peng, C.; Zhou, X.; Chen, L.; Wang, W.; Zhang, Y.; Ma, P.X.; He, C. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon 2017, 116, 325–337. [Google Scholar] [CrossRef]
- Wu, C.; Xia, L.; Han, P.; Xu, M.; Fang, B.; Wang, J.; Chang, J.; Xiao, Y. Graphene-oxide-modified β-tricalcium phosphate bioceramics stimulate in vitro and in vivo osteogenesis. Carbon 2015, 93, 116–129. [Google Scholar] [CrossRef]
- Pandele, A.M.; Ionita, M.; Lungu, A.; Vasile, E.; Zaharia, C.; Iovu, H. Porous chitosan/graphene oxide biocomposites for tissue engineering. Polym. Compos. 2017, 38, 363–370. [Google Scholar] [CrossRef]
- Hermenean, A.; Codreanu, A.; Herman, H.; Balta, C.; Rosu, M.; Mihali, C.V.; Ivan, A.; Dinescu, S.; Ionita, M.; Costache, M. Chitosan-Graphene Oxide 3D scaffolds as Promising Tools for Bone Regeneration in Critical-Size Mouse Calvarial Defects. Sci. Rep. 2017, 7, 16641. [Google Scholar] [CrossRef]
- Vera-Sánchez, M.; Aznar-Cervantes, S.; Jover, E.; García-Bernal, D.; Oñate-Sánchez, R.E.; Hernández-Romero, D.; Moraleda, J.M.; Collado-González, M.; Rodríguez-Lozano, F.J.; Cenis, J.L. Silk-Fibroin and Graphene Oxide Composites Promote Human Periodontal Ligament Stem Cell Spontaneous Differentiation into Osteo/Cementoblast-Like Cells. Stem Cells Dev. 2016, 25, 1742–1754. [Google Scholar] [CrossRef]
- Torii, D.; Tsutsui, T.W.; Watanabe, N.; Konishi, K. Bone morphogenetic protein 7 induces cementogenic differentiation of human periodontal ligament-derived mesenchymal stem cells. Odontology 2016, 104, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Su, G.; Sun, X.; Wang, Y.; Fang, Z.; Chen, M.; Zhang, Q. Graphene Oxide Incorporated Collagen/Nano-Hydroxyapatite Composites with Improved Mechanical Properties for Bone Repair Materials. J. Biomater. Tissue Eng. 2017, 7, 1000–1007. [Google Scholar] [CrossRef]
- Rajan Unnithan, A.; Ramachandra Kurup Sasikala, A.; Park, C.H.; Kim, C.S. A unique scaffold for bone tissue engineering: An osteogenic combination of graphene oxide–hyaluronic acid–chitosan with simvastatin. J. Ind. Eng. Chem. 2017, 46, 182–191. [Google Scholar] [CrossRef]
- Saravanan, S.; Chawla, A.; Vairamani, M.; Sastry, T.P.; Subramanian, K.S.; Selvamurugan, N. Scaffolds containing chitosan, gelatin and graphene oxide for bone tissue regeneration in vitro and in vivo. Int. J. Biol. Macromol. 2017, 104, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, H.; He, F.; Wu, T.; Zhou, L.; Ye, J. Concentration-dependent osteogenic and angiogenic biological performances of calcium phosphate cement modified with copper ions. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1199–1212. [Google Scholar] [CrossRef]
- Kawamoto, K.; Miyaji, H.; Nishida, E.; Miyata, S.; Kato, A.; Tateyama, A.; Furihata, T.; Shitomi, K.; Iwanaga, T.; Sugaya, T. Characterization and evaluation of graphene oxide scaffold for periodontal wound healing of class II furcation defects in dog. Int. J. Nanomed. 2018, 13, 2365–2376. [Google Scholar] [CrossRef]
- Crowder, S.W.; Prasai, D.; Rath, R.; Balikov, D.A.; Bae, H.; Bolotin, K.I.; Sung, H.-J. Three-dimensional graphene foams promote osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2013, 5, 4171–4176. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Kim, J.E.; Jang, K.J.; Seonwoo, H.; Chung, J.H. Enhanced Osteogenic Differentiation of Periodontal Ligament Stem Cells Using a Graphene Oxide-Coated Poly(ε-caprolactone) Scaffold. Polymers 2021, 13, 797. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.C.; Lee, S.M.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Jeong, C.M.; Huh, J.B.; Han, D.W. Enhanced Osteogenesis by Reduced Graphene Oxide/Hydroxyapatite Nanocomposites. Sci. Rep. 2015, 5, 18833. [Google Scholar] [CrossRef]
- Shao, W.; He, J.; Sang, F.; Wang, Q.; Chen, L.; Cui, S.; Ding, B. Enhanced bone formation in electrospun poly(L-lactic-co-glycolic acid)-tussah silk fibroin ultrafine nanofiber scaffolds incorporated with graphene oxide. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 823–834. [Google Scholar] [CrossRef]
- Kim, J.W.; Shin, Y.C.; Lee, J.J.; Bae, E.B.; Jeon, Y.C.; Jeong, C.M.; Yun, M.J.; Lee, S.H.; Han, D.W.; Huh, J.B. The Effect of Reduced Graphene Oxide-Coated Biphasic Calcium Phosphate Bone Graft Material on Osteogenesis. Int. J. Mol. Sci. 2017, 18, 1725. [Google Scholar] [CrossRef]
- Han, L.; Sun, H.; Tang, P.; Li, P.; Xie, C.; Wang, M.; Wang, K.; Weng, J.; Tan, H.; Ren, F.; et al. Mussel-inspired graphene oxide nanosheet-enwrapped Ti scaffolds with drug-encapsulated gelatin microspheres for bone regeneration. Biomater. Sci. 2018, 6, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, V.; Reina, G.; Orecchioni, M.; Furesi, G.; Thiele, S.; Gardin, C.; Zavan, B.; Cuniberti, G.; Bianco, A.; Rauner, M.; et al. Stimulation of bone formation by monocyte-activator functionalized graphene oxide in vivo. Nanoscale 2019, 11, 19408–19421. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, C.; Mou, S.; Li, J.; Zhou, M.; Zeng, Y.; Luo, C.; Sun, J.; Wang, Z.; Xu, W. Biocompatible graphene oxide-collagen composite aerogel for enhanced stiffness and in situ bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yu, P.; Shi, X.; Ling, T.; Zeng, W.; Chen, A.; Yang, W.; Zhou, Z. Hierarchically Porous Hydroxyapatite Hybrid Scaffold Incorporated with Reduced Graphene Oxide for Rapid Bone Ingrowth and Repair. ACS Nano 2019, 13, 9595–9606. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, A.; Liu, Y.; Jiao, D.; Zeng, D.; Wang, X.; Cao, L.; Jiang, X. Enhanced bone regeneration of the silk fibroin electrospun scaffolds through the modification of the graphene oxide functionalized by BMP-2 peptide. Int. J. Nanomed. 2019, 14, 733–751. [Google Scholar] [CrossRef] [PubMed]
- Amiryaghoubi, N.; Noroozi Pesyan, N.; Fathi, M.; Omidi, Y. Injectable thermosensitive hybrid hydrogel containing graphene oxide and chitosan as dental pulp stem cells scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 1338–1357. [Google Scholar] [CrossRef] [PubMed]
- Pathmanapan, S.; Periyathambi, P.; Anandasadagopan, S.K. Fibrin hydrogel incorporated with graphene oxide functionalized nanocomposite scaffolds for bone repair—In vitro and in vivo study. Nanomedicine 2020, 29, 102251. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Prema, D.; Venkataprasanna, K.S.; Balagangadharan, K.; Selvamurugan, N.; Venkatasubbu, G.D. Nanocomposite chitosan film containing graphene oxide/hydroxyapatite/gold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 154, 62–71. [Google Scholar] [CrossRef]
- Dubey, N.; Rajan, S.S.; Bello, Y.D.; Min, K.-S.; Rosa, V. Graphene nanosheets to improve physico-mechanical properties of bioactive calcium silicate cements. Materials 2017, 10, 606. [Google Scholar] [CrossRef]
- Liu, C.; Tan, D.; Chen, X.; Liao, J.; Wu, L. Research on Graphene and Its Derivatives in Oral Disease Treatment. Int. J. Mol. Sci. 2022, 23, 4737. [Google Scholar] [CrossRef]
- Sun, L.; Yan, Z.; Duan, Y.; Zhang, J.; Liu, B. Improvement of the mechanical, tribological and antibacterial properties of glass ionomer cements by fluorinated graphene. Dent. Mater. 2018, 34, e115–e127. [Google Scholar] [CrossRef]
- Farooq, I.; Ali, S.; Al-Saleh, S.; AlHamdan, E.M.; AlRefeai, M.H.; Abduljabbar, T.; Vohra, F. Synergistic effect of bioactive inorganic fillers in enhancing properties of dentin adhesives—A review. Polymers 2021, 13, 2169. [Google Scholar] [CrossRef]
- Chen, W.; Jin, H.; Zhang, H.; Wu, L.; Chen, G.; Shao, H.; Wang, S.; He, X.; Zheng, S.; Cao, C.Y. Synergistic effects of graphene quantum dots and carbodiimide in promoting resin–dentin bond durability. Dent. Mater. 2021, 37, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossù, M.; Ierardo, G.; Polimeni, A.; et al. Graphene-based dental adhesive with anti-biofilm activity. J. Nanobiotechnol. 2017, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Aati, S.; Clode, P.; Saunders, M.; Ngo, H.; Fawzy, A.S. Formulation of nano-graphene doped with nano silver modified dentin bonding agents with enhanced interfacial stability and antibiofilm properties. Dent. Mater. 2022, 38, 347–362. [Google Scholar] [CrossRef] [PubMed]
- AlFawaz, Y.F.; Almutairi, B.; Kattan, H.F.; Zafar, M.S.; Farooq, I.; Naseem, M.; Vohra, F.; Abduljabbar, T. Dentin bond integrity of hydroxyapatite containing resin adhesive enhanced with graphene oxide nano-particles—An SEM, EDX, micro-Raman, and microtensile bond strength study. Polymers 2020, 12, 2978. [Google Scholar] [CrossRef]
- Nahorny, S.; Zanin, H.; Christino, V.A.; Marciano, F.R.; Lobo, A.O.; Soares, L.E.S. Multi-walled carbon nanotubes/graphene oxide hybrid and nanohydroxyapatite composite: A novel coating to prevent dentin erosion. Mater. Sci. Eng. C 2017, 79, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Nizami, M.Z.I.; Nishina, Y.; Yamamoto, T.; Shinoda-Ito, Y.; Takashiba, S. Functionalized graphene oxide shields tooth dentin from decalcification. J. Dent. Res. 2020, 99, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Fallahzadeh, F.; Safarzadeh-Khosroshahi, S.; Atai, M. Dentin bonding agent with improved bond strength to dentin through incorporation of sepiolite nanoparticles. J. Clin. Exp. Dent. 2017, 9, e738. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, A.; Bin-Shuwaish, M.S.; Al-Hamdan, R.S.; Almohareb, T.; Maawadh, A.M.; Al Deeb, M.; Alhenaki, A.M.; Abduljabbar, T.; Vohra, F. Graphene oxide nano-filler based experimental dentine adhesive. A SEM/EDX, Micro-Raman and microtensile bond strength analysis. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020966936. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent. Mater. 2014, 30, 182–191. [Google Scholar] [CrossRef]
- Xie, H.; Cao, T.; Rodríguez-Lozano, F.J.; Luong-Van, E.K.; Rosa, V. Graphene for the development of the next-generation of biocomposites for dental and medical applications. Dent. Mater. 2017, 33, 765–774. [Google Scholar] [CrossRef]
- Astudillo-Rubio, D.; Delgado-Gaete, A.; Bellot-Arcís, C.; Montiel-Company, J.M.; Pascual-Moscardó, A.; Almerich-Silla, J.M. Mechanical properties of provisional dental materials: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0193162. [Google Scholar] [CrossRef]
- Kim, S.H.; Watts, D.C. Effect of glass-fiber reinforcement and water storage on fracture toughness (KIC) of polymer-based provisional crown and FPD materials. Int. J. Prosthodont. 2004, 17, 318–322. [Google Scholar] [PubMed]
- Samadzadeh, A.; Kugel, G.; Hurley, E.; Aboushala, A. Fracture strengths of provisional restorations reinforced with plasma-treated woven polyethylene fiber. J. Prosthet. Dent. 1997, 78, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Tanculescu, O.; Doloca, A.; Vieriu, R.M.; Mocanu, F.; Iovan, G.; Ifteni, G.; Martu, S.; Ioanid, N. Physical and Mechanical Characterization of Different Fiber-reinforced Composite Systems Used in Fixed Prosthesis. Rev. Chim. 2016, 67, 96–102. [Google Scholar]
- Malik, S.; Ruddock, F.M.; Dowling, A.H.; Byrne, K.; Schmitt, W.; Khalakhan, I.; Nemoto, Y.; Guo, H.; Shrestha, L.K.; Ariga, K.; et al. Graphene composites with dental and biomedical applicability. Beilstein J. Nanotechnol. 2018, 9, 801–808. [Google Scholar] [CrossRef] [PubMed]
- El Achaby, M.; Arrakhiz, F.Z.; Vaudreuil, S.; Essassi, E.M.; Qaiss, A. Piezoelectric β-polymorph formation and properties enhancement in graphene oxide—PVDF nanocomposite films. Appl. Surf. Sci. 2012, 258, 7668–7677. [Google Scholar] [CrossRef]
- Kumar, S.; Raj, S.; Kolanthai, E.; Sood, A.K.; Sampath, S.; Chatterjee, K. Chemical Functionalization of Graphene To Augment Stem Cell Osteogenesis and Inhibit Biofilm Formation on Polymer Composites for Orthopedic Applications. ACS Appl. Mater. Interfaces 2015, 7, 3237–3252. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Srivastava, I.; Wang, Z.; Song, H.; Yu, Z.-Z.; Koratkar, N. Fracture and Fatigue in Graphene Nanocomposites. Small 2010, 6, 179–183. [Google Scholar] [CrossRef]
- Martínez, J.M.A.; Moyano, M.A.; Castillo, M.Á.S.; Pellicer, A. Procedimiento para la Preparación de Resinas Polimerizables con Derivados de Grafeno. Spain Patent ES2547476B1, 26 September 2016. [Google Scholar]
- Lee, J.H.; Jo, J.K.; Kim, D.A.; Patel, K.D.; Kim, H.W.; Lee, H.H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Azevedo, L.; Antonaya-Martin, J.L.; Molinero-Mourelle, P.; Del Río-Highsmith, J. Improving PMMA resin using graphene oxide for a definitive prosthodontic rehabilitation—A clinical report. J. Clin. Exp. Dent. 2019, 11, e670–e674. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The Influence of Graphene in Improvement of Physico-Mechanical Properties in PMMA Denture Base Resins. Materials 2019, 12, 2335. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.; Marques, P.A.A.P.; Barros-Timmons, A.; Bdkin, I.; Singh, M.K.; Emami, N.; Grácio, J. Graphene oxide modified with PMMA via ATRP as a reinforcement filler. J. Mater. Chem. 2010, 20, 9927–9934. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/Polymer Nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.C.; Paolone, G.; Gherlone, E.; Ferracane, J.L.; Brambilla, E. In vitro biofilm formation on resin-based composites after different finishing and polishing procedures. J. Dent. 2017, 67, 43–52. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Sauro, S.; Pires, P.M.; Lòpez-Castellano, A.; Alambiaga-Caravaca, A.M.; Brambilla, E. Antimicrobial properties of PMMA resin containing graphene. Dent. Mater. 2019, 35, e48. [Google Scholar] [CrossRef]
- Khalichi, P.; Cvitkovitch, D.G.; Santerre, J.P. Effect of composite resin biodegradation products on oral streptococcal growth. Biomaterials 2004, 25, 5467–5472. [Google Scholar] [CrossRef]
- Surnova, A.; Balkaev, D.; Musin, D.; Amirov, R.; Dimiev, A.M. Fully exfoliated graphene oxide accelerates epoxy resin curing, and results in dramatic improvement of the polymer mechanical properties. Compos. Part B Eng. 2019, 162, 685–691. [Google Scholar] [CrossRef]
- Tripathi, S.N.; Saini, P.; Gupta, D.; Choudhary, V. Electrical and mechanical properties of PMMA/reduced graphene oxide nanocomposites prepared via in situ polymerization. J. Mater. Sci. 2013, 48, 6223–6232. [Google Scholar] [CrossRef]
- Rajesh, A.; Mangamma, G.; Sairam, T.N.; Subramanian, S.; Kalavathi, S.; Kamruddin, M.; Dash, S. Physicochemical properties of nanocomposite: Hydroxyapatite in reduced graphene oxide. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 203–210. [Google Scholar] [CrossRef]
- Shi, L.; Bai, Y.; Su, J.; Ma, W.; Jia, R.-l. Graphene oxide/fluorhydroxyapatite composites with enhanced chemical stability, mechanical, and biological properties for dental applications. Int. J. Appl. Ceram. Technol. 2017, 14, 1088–1100. [Google Scholar] [CrossRef]
- Nam, H.J.; Kim, Y.M.; Kwon, Y.H.; Kim, I.R.; Park, B.S.; Son, W.S.; Lee, S.M.; Kim, Y.I. Enamel Surface Remineralization Effect by Fluorinated Graphite and Bioactive Glass-Containing Orthodontic Bonding Resin. Materials 2019, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, S.; De Angelis, F.; Brauner, E.; Pranno, N.; Tassi, G.; Senatore, M.; Bossù, M. Flexural strength and elastic modulus evaluation of structures made by conventional PMMA and PMMA reinforced with graphene. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5201–5208. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Shetty, S. Effect of Addition of Graphene and Carbon Nanotubes on Flexural Strength of Polymethylmethacrylate- A Comparative In-Vitro Study. J. Evol. Med. Dent. Sci. 2020, 9, 1494–1499. [Google Scholar] [CrossRef]
- Jiang, N.; Tan, P.; He, M.; Zhang, J.; Sun, D.; Zhu, S. Graphene reinforced polyether ether ketone nanocomposites for bone repair applications. Polym. Test. 2021, 100, 107276. [Google Scholar] [CrossRef]
- Levenez, B.; Gil-Cortes, T.; Rodríguez-Fuentes, N.; Jiménez, J.E.; Herrera-Kao, W.; Loría-Bastarrachea, M.I.; May-Pat, A.; Guerrero-Bermea, C.; Uribe-Calderón, J.; Cervantes-Uc, J.M. Silanized graphene oxide as a reinforcing agent for acrylic bone cements: Physicochemical, mechanical and biological characterization. J. Biomater. Sci. Polym. Ed. 2021, 32, 1736–1753. [Google Scholar] [CrossRef]
- Ciocan, L.T.; Ghitman, J.; Vasilescu, V.G.; Iovu, H. Mechanical Properties of Polymer-Based Blanks for Machined Dental Restorations. Materials 2021, 14, 7293. [Google Scholar] [CrossRef]
- Çakmak, G.; Donmez, M.B.; Akay, C.; Abou-Ayash, S.; Schimmel, M.; Yilmaz, B. Effect of Thermal Cycling on the Flexural Strength and Hardness of New-Generation Denture Base Materials. J. Prosthodont. 2023, 32, 81–86. [Google Scholar] [CrossRef]
- Aati, S.; Chauhan, A.; Shrestha, B.; Rajan, S.M.; Aati, H.; Fawzy, A. Development of 3D printed dental resin nanocomposite with graphene nanoplatelets enhanced mechanical properties and induced drug-free antimicrobial activity. Dent. Mater. 2022, 38, 1921–1933. [Google Scholar] [CrossRef]
- Khan, A.A.; De Vera, M.A.T.; Mohamed, B.A.; Javed, R.; Al-Kheraif, A.A. Enhancing the physical properties of acrylic resilient denture liner using graphene oxide nanosheets. J. Vinyl Addit. Technol. 2022, 28, 487–493. [Google Scholar] [CrossRef]
- Velo, M.; Filho, F.G.N.; de Lima Nascimento, T.R.; Obeid, A.T.; Castellano, L.C.; Costa, R.M.; Brondino, N.C.M.; Fonseca, M.G.; Silikas, N.; Mondelli, R.F.L. Enhancing the mechanical properties and providing bioactive potential for graphene oxide/montmorillonite hybrid dental resin composites. Sci. Rep. 2022, 12, 10259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tremblay, P.-L. Graphene: An Antibacterial Agent or a Promoter of Bacterial Proliferation? iScience 2020, 23, 101787. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Z.; Chen, C.; Lynch, I. Uncertainties in the antibacterial mechanisms of graphene family materials. Nano Today 2022, 43, 101436. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, H.; von dem Bussche, A.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar] [CrossRef]
- Volkov, Y.; McIntyre, J.; Prina-Mello, A. Graphene toxicity as a double-edged sword of risks and exploitable opportunities: A critical analysis of the most recent trends and developments. 2D Materials 2017, 4, 022001. [Google Scholar] [CrossRef]
- Singh, N.; Manshian, B.; Jenkins, G.J.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef]
- Park, C.; Park, S.; Lee, D.; Choi, K.S.; Lim, H.P.; Kim, J. Graphene as an Enabling Strategy for Dental Implant and Tissue Regeneration. Tissue Eng. Regen. Med. 2017, 14, 481–493. [Google Scholar] [CrossRef]
- Tonelli, F.M.; Goulart, V.A.; Gomes, K.N.; Ladeira, M.S.; Santos, A.K.; Lorençon, E.; Ladeira, L.O.; Resende, R.R. Graphene-based nanomaterials: Biological and medical applications and toxicity. Nanomedicine 2015, 10, 2423–2450. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Dziewięcka, M.; Pawlyta, M.; Majchrzycki, Ł.; Balin, K.; Barteczko, S.; Czerkawska, M.; Augustyniak, M. The Structure–Properties–Cytotoxicity Interplay: A Crucial Pathway to Determining Graphene Oxide Biocompatibility. Int. J. Mol. Sci. 2021, 22, 5401. [Google Scholar] [CrossRef] [PubMed]
- Neuss, S.; Apel, C.; Buttler, P.; Denecke, B.; Dhanasingh, A.; Ding, X.; Grafahrend, D.; Groger, A.; Hemmrich, K.; Herr, A.; et al. Assessment of stem cell/biomaterial combinations for stem cell-based tissue engineering. Biomaterials 2008, 29, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotechnol. 2020, 8, 465. [Google Scholar] [CrossRef]
- Silva, R.C.S.; Agrelli, A.; Andrade, A.N.; Mendes-Marques, C.L.; Arruda, I.R.S.; Santos, L.R.L.; Vasconcelos, N.F.; Machado, G. Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections. Materials 2022, 15, 3150. [Google Scholar] [CrossRef]
- Purohit, S.D.; Bhaskar, R.; Singh, H.; Yadav, I.; Gupta, M.K.; Mishra, N.C. Development of a nanocomposite scaffold of gelatin-alginate-graphene oxide for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 592–602. [Google Scholar] [CrossRef]
- Wang, W.; Junior, J.R.P.; Nalesso, P.R.L.; Musson, D.; Cornish, J.; Mendonça, F.; Caetano, G.F.; Bártolo, P. Engineered 3D printed poly(ɛ-caprolactone)/graphene scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 759–770. [Google Scholar] [CrossRef]
- Medeiros, J.S.; Oliveira, A.M.; Carvalho, J.O.; Ricci, R.; Martins, M.; Rodrigues, B.V.M.; Webster, T.J.; Viana, B.C.; Vasconcellos, L.M.R.; Canevari, R.A.; et al. Nanohydroxyapatite/Graphene Nanoribbons Nanocomposites Induce in Vitro Osteogenesis and Promote in Vivo Bone Neoformation. ACS Biomater. Sci. Eng. 2018, 4, 1580–1590. [Google Scholar] [CrossRef]
- Cai, X.; Tan, S.; Yu, A.; Zhang, J.; Liu, J.; Mai, W.; Jiang, Z. Sodium 1-naphthalenesulfonate-functionalized reduced graphene oxide stabilizes silver nanoparticles with lower cytotoxicity and long-term antibacterial activity. Chem. Asian J. 2012, 7, 1664–1670. [Google Scholar] [CrossRef]
- Vuppaladadium, S.S.R.; Agarwal, T.; Kulanthaivel, S.; Mohanty, B.; Barik, C.S.; Maiti, T.K.; Pal, S.; Pal, K.; Banerjee, I. Silanization improves biocompatibility of graphene oxide. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110647. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Kim, H.D.; Han, S.S. Biocompatibility and hemocompatibility of hydrothermally derived reduced graphene oxide using soluble starch as a reducing agent. Colloids Surf. B Biointerfaces 2020, 185, 110579. [Google Scholar] [CrossRef] [PubMed]
- Sirajunisha, H.; Sakthivel, P.; Balakrishnan, T. Structural, photoluminescence, antibacterial and biocompatibility features of zinc incorporated hydroxyapatite nanocomposites. J. Mater. Sci. Mater. Electron. 2021, 32, 5050–5064. [Google Scholar] [CrossRef]







| Inclusion Criteria | Exclusion Criteria |
|---|---|
| English language In vitro studies In vivo studies Research focused on dental biomaterials used for direct and indirect dental restorations, as well as periodontal treatment | Full text is not available Review articles Opinion articles Nonprimary research Studies on graphene materials that are not for oral or dental applications Studies on biomaterials used in dental fields other than restorative and periodontal dentistry |
| Material | Pathogen | Antimicrobial Outcomes | References |
|---|---|---|---|
| GO | E. coli | Antimicrobial activity by insertion, edge-cutting, and lipid extraction | Tu et al., 2013 [82] |
| Ag/GNP | E. coli | Antimicrobial activity by cell entrapment | Vi et al., 2018 [83] |
| Ag-rGO | E. coli | Antimicrobial activity by cell entrapment, cell membrane damage, oxidative stress, and the bactericidal action of Ag+ | Moghayedi et al., 2017 [84] |
| GMgO-Ag | E. coli | Antimicrobial activity caused by membrane damage | Zhang et al., 2016 [85] |
| GQD | E. coli | Antimicrobial activity by ROS generation, photoexcited killing, and cell membrane damage | Ristic et al., 2014 [86] |
| GO | E. coli | Antimicrobial activity | Aunkor et al., 2020 [87] |
| GO and rGO-poly(dopamine) | S. aureus | Antimicrobial activity by cell membrane damage, ROS generation, and electron transfer | Jia et al., 2016 [88] |
| GO-AgNPs | S. aureus | Antimicrobial activity by cell membrane damage and ROS generation | Jaworski et al., 2018 [89] |
| PLGA/chitosan/GO/AgNPs | S. aureus | Antimicrobial activity by catalytic oxidation by silver, cell membrane damage, and ROS generation | De Faria et al., 2015 [90] |
| rGO/Ag | S. mutans | Antimicrobial activity by cell entrapment and the bactericidal action of Ag+ | Wu et al., 2019 [91] |
| GO | S. mutans | Up to 80% antimicrobial activity | Yu et al., 2020 [92] |
| Nano-graphene oxide with antisense vicR RNA plasmid | S. mutans | Reduced virulent-associated gene expressions, suppressed biofilm aggregation, and inhibited EPS accumulation | Wu et al., 2020 [93] |
| GO/AgNPs | C. albicans | Antimicrobial activity by cell membrane damage and oxidative stress | Jaworski et al., 2018 [89] |
| GO | E. coli S. aureus | Antimicrobial activity by disruption of bacterial cellular membranes | Farid et al., 2018 [69] |
| Nanographene oxide | P. gingivalis | Biofilm and bacterial metabolism reduction | Pourhajibagher et al., 2022 [77] |
| GO | S. mutans F. nucleatum P.gingivalis | Antimicrobial activity by cell membrane damage | He et al., 2015 [68] |
| rGNs/Ag | C. albicans L. acidophilus S. mutans A. actinomycetemcomitans | Higher antimicrobial properties than R-GN and AgNPs alone | Peng et al., 2017 [70] |
| Ti/GO/Ag | S. aureus S. mutans P. gingivalis | Antimicrobial activity by bacterial cell shrinking, perforation, breaking, and bursting | Jin et al., 2017 [71] |
| GO | S. mutans; P. gingivalis; F. nucleatum | Elimination of residual bacteria and inhibition of biofilm reformation | Qin et al., 2020 [73] |
| G/AgNp | S. aureus S. mutans E. coli | Antibacterial activity | Bacali et al., 2020 [94] |
| Ti/0.125G | S. mutans F. nucleatum P. gingivalis | Suppressed bacterial growth | Wei et al., 2021 [72] |
| PEEK/GO | S. mutans F. nucleatum P. gingivalis | Bacterial inhibition | Guo et al., 2021 [75] |
| Ti/6Al/4V | C. albicans P. gingivalis F. nucleatum | Bacterial inhibition by ROS generation | Wang et al., 2022 [74] |
| GQD | A. actinomycetemcomitans P. gingivalis P. intermedia | Bacterial inhibition by ROS generation | Pourhajibagher et al., 2019 [80] |
| Material | Effect | References |
|---|---|---|
| rGO/HA nanocomposites | Increased ALP; mineralization; and osteopontin; osteocalcin expression | Lee et al., 2015 [182] |
| Poly(L-lactic-co-glycolic acid) with Tussah silk fibroin; GO | High adhesion; proliferation; ALP; mineral deposition | Shao et al., 2016 [183] |
| Silk-fibroin/GO | Osteogenic and cementoblast differentiation | Vera-Sánchez et al., 2016 [173] |
| CaP/rGO | Accelerated bone neo-formation | Kim et al., 2017 [184] |
| Collagen-GO membrane | Roughness and stiffness; osteogenic differentiation | Marco et al. 2017 [158] |
| Ti/GO/BMP-2/vancomycin | Osteogenic activity | Han et al., 2018 [185] |
| 3D collagen sponge/GO | Osteogenic differentiation; PDL-like and cementum-like tissue regeneration | Kawamoto et al., 2018 [179] |
| monocytes activator GO complexed with CaP | Activation of monocytes; stimulated osteogenesis | Bordoni et al., 2019 [186] |
| GO-collagen aerogel | Biomineralization; biocompatibility; osteogenic activity | Liu et al., 2019 [187] |
| HA/rGO | Proliferation; osteogenic activity | Zhou et al., 2019 [188] |
| Silk fibrinoid/GO/BMP-2 | Biocompatibility; adhesion; proliferation; osteogenic differentiation | Wu et al., 2019 [189] |
| GO/chitosan | Osteogenic differentiation | Amiryaghoubi et al. (2020) [190] |
| GO/IONPs/H | Biocompatible; osteogenic activity; calcium deposits | Pathmanapan et al., 2020 [191] |
| GO/HA/Au | Biocompatibility; osteogenic differentiation | Prakash et al., 2020 [192] |
| Material | Effect | References |
|---|---|---|
| PMMA/rGO—incorporated into the liquid | High concentrations decreased PMMA tensile strength. Lower concentrations exhibited no changes | Tripathi et al., 2013 [226] |
| Gp-NSs | Improved physical-mechanical properties of bioactive cement | Dubey et al., 2017 [193] |
| nHA/MWCNTO/GO | Formation of a protective layer for dentin against erosive processes | Nahorny et al., 2017 [201] |
| rGO-HA | The elasticity has improved tenfold compared with that of HA | Rajesh et al., 2017 [227] |
| GO-based fluorhydroxyapatite | Enamel and dentin mineralization | Shi et al., 2017 [228] |
| PMMA/GO—incorporated into the liquid | GO-concentrations ≥ 0.5 wt% increased the PMMA hardness and flexural strength | Lee et al., 2018 [216] |
| Fluorinated graphene | Increased microhardness and compressive strength; decreased friction coefficient | Sun et al., 2018 [195] |
| PMMA/GO—Commercial CAD-CAM resin block | GO incorporation into PMMA did not influence the hardness or flexural strength | Agarwalla et al., 2019 [40] |
| G/AgNp | Minimal toxicity and improved flexural properties | Bacali et al., 2019 [219] |
| GO | Enhanced shear bond strength | Khan et al., 2019 [152] |
| Graphite Fluoride bioactive glass | Enamel and dentin mineralization | Nam et al., 2019 [229] |
| PMMA/GO—Commercial CAD-CAM resin block | Increased flexural strength | Di Carlo et al., 2020 [230] |
| PMMA/GO—incorporated into the liquid | Decreased flexural strength | Ghosh and Shetty, 2020 [231] |
| PEEK/GNP—injection molding | Higher flexural, tensile, and compression strength | Jiang et al., 2021 [232] |
| Bone cement PMMA based/GO incorporated into the liquid | Increased bone cement compression strength | Levenez et al., 2021 [233] |
| PMMA/GO—Commercial CAD-CAM resin block | Decreased hardness | Ciocan et al., 2021 [234] |
| PMMA/GO—Commercial CAD-CAM resin block | Increased flexural strength | Çakmak et al., 2022 [235] |
| PMMA/GNP—3D printed resin | Improved strength, hardness, and elasticity; antimicrobial activity | Aati et al., 2022 [236] |
| Soft denture liner PMMA based/GO—incorporated into the liquid | No influence on denture liner hardness | Khan et al., 2022 [237] |
| GO/montmorillonite | A more stable compound; enamel and dentin mineralization | Velo et al., 2022 [238] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostu, A.M.; Sufaru, I.-G.; Tanculescu, O.; Stoleriu, S.; Doloca, A.; Ciocan Pendefunda, A.A.; Solomon, S.M. Can Graphene Pave the Way to Successful Periodontal and Dental Prosthetic Treatments? A Narrative Review. Biomedicines 2023, 11, 2354. https://doi.org/10.3390/biomedicines11092354
Apostu AM, Sufaru I-G, Tanculescu O, Stoleriu S, Doloca A, Ciocan Pendefunda AA, Solomon SM. Can Graphene Pave the Way to Successful Periodontal and Dental Prosthetic Treatments? A Narrative Review. Biomedicines. 2023; 11(9):2354. https://doi.org/10.3390/biomedicines11092354
Chicago/Turabian StyleApostu, Alina Mihaela, Irina-Georgeta Sufaru, Oana Tanculescu, Simona Stoleriu, Adrian Doloca, Alice Arina Ciocan Pendefunda, and Sorina Mihaela Solomon. 2023. "Can Graphene Pave the Way to Successful Periodontal and Dental Prosthetic Treatments? A Narrative Review" Biomedicines 11, no. 9: 2354. https://doi.org/10.3390/biomedicines11092354