Spontaneous Neutrophil Extracellular Traps Release Are Inflammatory Markers Associated with Hyperglycemia and Renal Failure on Diabetic Retinopathy
Abstract
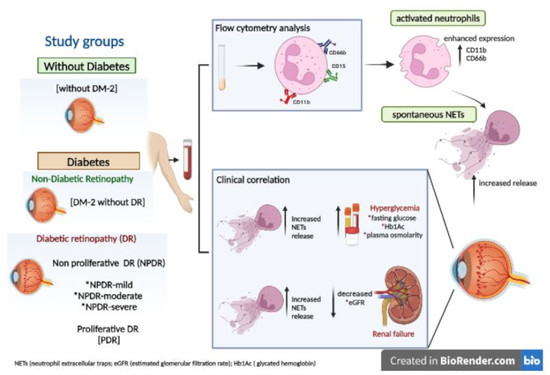
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Participants
2.3. Flow Cytometry Assays
2.4. Peripheral Blood Polymorphonuclear (PMN) Cell Isolation
2.5. Microscopy Staining of In Vitro NETs
2.6. Image NET Analysis
2.7. Sample Size Calculation
2.8. Statistical Analysis
3. Results
3.1. Increased Activated Neutrophil Markers and Spontaneous NET Release Relationship with the Progression Time of Diabetes in Severe Stages of DR
3.2. NETs Correlated with the Hyperglycemic and Renal Status on Severe Stages of DR
3.3. Risk Prognosis of DR Development by Association of the Spontaneous NET Release
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barquera, S.; Pedroza-Tobias, A.; Medina, C. Cardiovascular diseases in mega-countries: The challenges of the nutrition, physical activity and epidemiologic transitions, and the double burden of disease. Curr. Opin. Lipidol. 2016, 27, 329–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L. Classification of diabetic retinopathy and diabetic macular edema. World J. Diabetes 2013, 4, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.; O’Leary, O.E.; Stitt, A.W. The pathology associated with diabetic retinopathy. Vis. Res. 2017, 139, 7–14. [Google Scholar] [CrossRef]
- Milne, R.; Brownstein, S. Advanced glycation end products and diabetic retinopathy. Amino Acids 2011, 44, 1397–1407. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Rada, B. Neutrophil Extracellular Traps. Methods Mol. Biol. 2019, 1982, 517–528. [Google Scholar]
- Cappenberg, A.; Kardell, M.; Zarbock, A. Selectin-Mediated Signaling—Shedding Light on the Regulation of Integrin Activity in Neutrophils. Cells 2022, 11, 1310. [Google Scholar] [CrossRef]
- Zhou, H.; Liao, J.; Aloor, J.; Nie, H.; Wilson, B.C.; Fessler, M.B.; Gao, H.-M.; Hong, J.-S. CD11b/CD18 (Mac-1) Is a Novel Surface Receptor for Extracellular Double-Stranded RNA To Mediate Cellular Inflammatory Responses. J. Immunol. 2013, 190, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Czaikoski, P.G.; Mota, J.M.S.C.; Nascimento, D.C.; Sônego, F.; Castanheira, F.V.E.S.; Melo, P.H.; Scortegagna, G.T.; Silva, R.L.; Barroso-Sousa, R.; Souto, F.O.; et al. Neutrophil Extracellular Traps Induce Organ Damage during Experimental and Clinical Sepsis. PLoS ONE 2016, 11, e0148142. [Google Scholar] [CrossRef] [Green Version]
- Perdomo, J.; Leung, H.H.; Ahmadi, Z.; Yan, F.; Chong, J.J.; Passam, F.H.; Chong, B.H. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocyto-penia. Nat. Commun. 2019, 10, 1322. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J. Cell Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef] [Green Version]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef] [Green Version]
- Leffler, J.; Martin, M.; Gullstrand, B.; Tydén, H.; Lood, C.; Truedsson, L.; Bengtsson, A.A.; Blom, A.M. Neutrophil Extracellular Traps That Are Not Degraded in Systemic Lupus Erythematosus Activate Complement Exacerbating the Disease. J. Immunol. 2012, 188, 3522–3531. [Google Scholar] [CrossRef] [Green Version]
- Dicker, A.J.; Crichton, M.L.; Pumphrey, E.G.; Cassidy, A.J.; Suarez-Cuartin, G.; Sibila, O.; Furrie, E.; Fong, C.J.; Ibrahim, W.; Brady, G.; et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Gupta, E.; Kaushik, S.; Jyoti, A. Neutrophil Extracellular Traps: Formation and Involvement in Disease Progression. Iran. J. Allergy Asthma Immunol. 2018, 17, 208–220. [Google Scholar]
- Seo, J.D.; Gu, J.-Y.; Jung, H.S.; Kim, Y.J.; Kim, H.K. Contact System Activation and Neutrophil Extracellular Trap Markers: Risk Factors for Portal Vein Thrombosis in Patients With Hepatocellular Carcinoma. Clin. Appl. Thromb. 2019, 25, 1076029618825310. [Google Scholar] [CrossRef] [Green Version]
- Menegazzo, L.; Ciciliot, S.; Poncina, N.; Mazzucato, M.; Persano, M.; Bonora, B.; Albiero, M.; de Kreutzenberg, S.V.; Avogaro, A.; Fadini, G.P. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. 2014, 52, 497–503. [Google Scholar] [CrossRef]
- Carestia, A.; Frechtel, G.; Cerrone, G.; Linari, M.A.; Gonzalez, C.; Casais, P.; Schattner, M. NETosis before and after Hyperglycemic Control in Type 2 Diabetes Mellitus Patients. PLoS ONE 2016, 11, e0168647. [Google Scholar] [CrossRef]
- Bryk, A.H.; Prior, S.M.; Plens, K.; Konieczynska, M.; Hohendorff, J.; Malecki, M.T.; Butenas, S.; Undas, A. Predictors of neutrophil extracellular traps markers in type 2 diabetes mellitus: Associations with a pro-thrombotic state and hypofibrinolysis. Cardiovasc. Diabetol. 2019, 18, 49. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, J.-E.; Gu, J.-Y.; Yoo, H.J.; Kim, Y.I.; Nam-Goong, I.S.; Kim, E.S.; Kim, H.K. Evaluation of Circulating Markers of Neutrophil Extracellular Trap (NET) Formation as Risk Factors for Diabetic Retinopathy in a Case-Control Association Study. Exp. Clin. Endocrinol. Diabetes 2016, 124, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, X.; Yin, Y.; Mai, Y.; Wang, D.; Zhang, X. Hyperglycemia Induces Neutrophil Extracellular Traps Formation Through an NADPH Oxidase-Dependent Pathway in Diabetic Retinopathy. Front. Immunol. 2019, 9, 3076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estúa-Acosta, G.A.; Buentello-Volante, B.; Magaña-Guerrero, F.S.; Flores, J.E.A.; Vivanco-Rojas, O.; Castro-Salas, I.; Zarco-Ávila, K.; García-Mejía, M.A.; Garfias, Y. Human Amniotic Membrane Mesenchymal Stem Cell-Synthesized PGE(2) Exerts an Immunomod-ulatory Effect on Neutrophil Extracellular Trap in a PAD-4-Dependent Pathway through EP2 and EP4. Cells 2022, 11, 2831. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Cavanaugh, L.; Leung, H.; Yan, F.; Ahmadi, Z.; Chong, B.H.; Passam, F. Quantification of NETs-associated markers by flow cytometry and serum assays in patients with thrombosis and sepsis. Int. J. Lab. Hematol. 2018, 40, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.B.; Ahamed, R.; Hegde, M.; Nair, A.S.; Ramachandra, L.; Satyamoorthy, K. Glucose induces metabolic reprogramming in neutrophils during type 2 diabetes to form constitutive extra-cellular traps and decreased responsiveness to lipopolysaccharides. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165940. [Google Scholar] [CrossRef]
- Freitas, D.F.; Colón, D.F.; Silva, R.L.; Santos, E.M.; Guimarães, V.H.D.; Ribeiro, G.H.M.; de Paula, A.M.B.; Guimarães, A.L.S.; dos Reis, S.T.; Cunha, F.Q.; et al. Neutrophil extracellular traps (NETs) modulate inflammatory profile in obese humans and mice: Adipose tissue role on NETs levels. Mol. Biol. Rep. 2022, 49, 3225–3236. [Google Scholar] [CrossRef]
- Shafqat, A.; Rab, S.A.; Ammar, O.; Al Salameh, S.; Alkhudairi, A.; Kashir, J.; Alkattan, K.; Yaqinuddin, A. Emerging role of neutrophil extracellular traps in the complications of diabetes mellitus. Front. Med. 2022, 9, 995993. [Google Scholar] [CrossRef]
- Lessieur, E.M.; Liu, H.; Saadane, A.; Du, Y.; Tang, J.; Kiser, J.; Kern, T.S. Neutrophil-Derived Proteases Contribute to the Pathogenesis of Early Diabetic Retinopathy. Investig. Opthalmology Vis. Sci. 2021, 62, 7. [Google Scholar] [CrossRef]
- Liu, H.; Lessieur, E.M.; Saadane, A.; Lindstrom, S.I.; Taylor, P.R.; Kern, T.S. Neutrophil elastase contributes to the pathological vascular permeability characteristic of diabetic retinopathy. Diabetologia 2019, 62, 2365–2374. [Google Scholar] [CrossRef]
- Van Oostrom, A.J.; Van Wijk, J.P.; Sijmonsma, T.P.; Rabelink, T.J.; Cabezas, M.C. Increased expression of activation markers on monocytes and neutrophils in type 2 diabetes. Neth. J. Med. 2004, 62, 320–325. [Google Scholar]
- Qiu, X.; Li, J.; Yang, X.; Tang, J.; Shi, J.; Tong, Y.; Qu, Y.; Mu, D. Is neutrophil CD11b a special marker for the early diagnosis of sepsis in neonates? A systematic review and meta-analysis. BMJ Open 2019, 9, e025222. [Google Scholar] [CrossRef] [Green Version]
- Quaas, A.; Pamuk, A.; Klein, S.; Quantius, J.; Rehkaemper, J.; Barutcu, A.G.; Rueschoff, J.; Zander, T.; Gebauer, F.; Hillmer, A.; et al. Sex-specific prognostic effect of CD66b-positive tumor-infiltrating neutrophils (TANs) in gastric and esoph-ageal adenocarcinoma. Gastric Cancer 2021, 24, 1213–1226. [Google Scholar] [CrossRef]
- Cong, X.; Zhang, Y.; Zhu, Z.; Li, S.; Yin, X.; Zhai, Z.; Zhang, Y.; Xue, Y. CD66b(+) neutrophils and alpha-SMA(+) fibroblasts predict clinical outcomes and benefits from postoperative chemotherapy in gastric adenocarcinoma. Cancer Med. 2020, 9, 2761–2773. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Nakagawa, T.; Sugihara, T.; Horiuchi, T.; Yoshizaki, U.; Fujimura, T.; Fukuhara, H.; Urano, T.; Takayama, K.; Inoue, S.; et al. Prognostic value of CD66b positive tumor-infiltrating neutrophils in testicular germ cell tumor. BMC Cancer 2016, 16, 898. [Google Scholar] [CrossRef] [Green Version]
- Sabbatini, M.; Bona, E.; Novello, G.; Migliario, M.; Renò, F. Aging hampers neutrophil extracellular traps (NETs) efficacy. Aging Clin. Exp. Res. 2022, 34, 2345–2353. [Google Scholar] [CrossRef]
- Wong, S.L.; Wagner, D.D. Peptidylarginine deiminase 4: A nuclear button triggering neutrophil extracellular traps in inflammatory diseases and aging. FASEB J. 2018, 32, 6258–6370. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, C.; Zou, Z.; Fan, E.K.Y.; Chen, L.; Li, Y.; Billiar, T.R.; Wilson, M.A.; Shi, X.; Fan, J. Aging-related Atg5 defect impairs neutrophil extracellular traps formation. Immunology 2017, 151, 417–432. [Google Scholar] [CrossRef] [Green Version]
- Mikhalchik, E.V.; Lipatova, V.A.; Basyreva, L.Y.; Panasenko, O.M.; Gusev, S.A.; Sergienko, V.I. Hyperglycemia and Some Aspects of Leukocyte Activation In Vitro. Bull. Exp. Biol. Med. 2021, 170, 748–751. [Google Scholar] [CrossRef]
- Martinez-Alberquilla, I.; Gasull, X.; Perez-Luna, P.; Seco-Mera, R.; Ruiz-Alcocer, J.; Crooke, A. Neutrophils and neutrophil extracellular trap components: Emerging biomarkers and thera-peutic targets for age-related eye diseases. Ageing Res. Rev. 2022, 74, 101553. [Google Scholar] [CrossRef]
- Arneth, B.; Arneth, R. Neutrophil Extracellular Traps (NETs) and Vasculitis. Int. J. Med. Sci. 2021, 18, 1532–1540. [Google Scholar] [CrossRef]
- Salazar-Gonzalez, H.; Zepeda-Hernandez, A.; Melo, Z.; Saavedra-Mayorga, D.E.; Echavarria, R. Neutrophil Extracellular Traps in the Establishment and Progression of Renal Diseases. Medicina 2019, 55, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langseth, M.S.; Opstad, T.B.; Bratseth, V.; Solheim, S.; Arnesen, H.; Pettersen, A.; Seljeflot, I.; Helseth, R. Markers of neutrophil extracellular traps are associated with adverse clinical outcome in stable coronary artery disease. Eur. J. Prev. Cardiol. 2018, 25, 762–769. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magaña-Guerrero, F.S.; Aguayo-Flores, J.E.; Buentello-Volante, B.; Zarco-Ávila, K.; Sánchez-Cisneros, P.; Castro-Salas, I.; De la Torre-Galván, E.; Rodríguez-Loaiza, J.L.; Jiménez-Corona, A.; Garfias, Y. Spontaneous Neutrophil Extracellular Traps Release Are Inflammatory Markers Associated with Hyperglycemia and Renal Failure on Diabetic Retinopathy. Biomedicines 2023, 11, 1791. https://doi.org/10.3390/biomedicines11071791
Magaña-Guerrero FS, Aguayo-Flores JE, Buentello-Volante B, Zarco-Ávila K, Sánchez-Cisneros P, Castro-Salas I, De la Torre-Galván E, Rodríguez-Loaiza JL, Jiménez-Corona A, Garfias Y. Spontaneous Neutrophil Extracellular Traps Release Are Inflammatory Markers Associated with Hyperglycemia and Renal Failure on Diabetic Retinopathy. Biomedicines. 2023; 11(7):1791. https://doi.org/10.3390/biomedicines11071791
Chicago/Turabian StyleMagaña-Guerrero, Fátima Sofía, José Eduardo Aguayo-Flores, Beatriz Buentello-Volante, Karla Zarco-Ávila, Paola Sánchez-Cisneros, Ilse Castro-Salas, Enya De la Torre-Galván, José Luis Rodríguez-Loaiza, Aida Jiménez-Corona, and Yonathan Garfias. 2023. "Spontaneous Neutrophil Extracellular Traps Release Are Inflammatory Markers Associated with Hyperglycemia and Renal Failure on Diabetic Retinopathy" Biomedicines 11, no. 7: 1791. https://doi.org/10.3390/biomedicines11071791