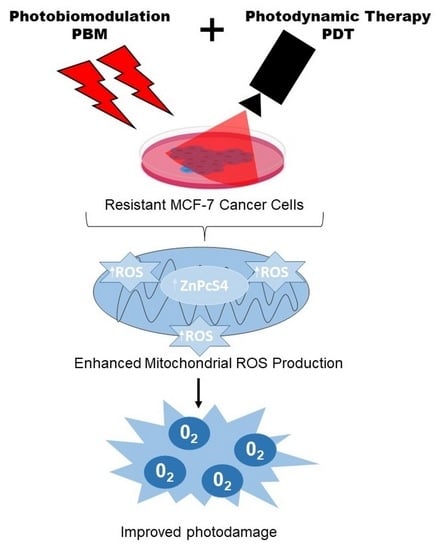
Photobiomodulation Improves Anti-Tumor Efficacy of Photodynamic Therapy against Resistant MCF-7 Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture Preparation
2.2. Photobiomodulation and Photodynamic Treatment
2.3. MTT Assay
2.4. Staining and Measurement of Mitochondrial Integrity with Rhodamine 123 (Rh-123)
2.5. ELISA Assay for Cytochrome c Protein Detection
2.6. Annexin V/Propidium Iodide (PI) Flow Cytometric Analysis of Cell Death
2.7. Fluorescence Measurement for Autophagy
2.8. Statistical Analysis
3. Results
3.1. Measurement of Cellular Viability
3.2. Assessment of Mitochondria Integrity with Rhodamine 123 Staining
3.3. ELISA Measurement of Cytochrome c Proteins Release
3.4. Annexin V/PI Staining
3.5. Analysis of Autophagy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Candido, N.M.; De Melo, M.T.; Franchi, L.P.; Primo, F.L.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Combining photodynamic therapy and chemotherapy: Improving breast cancer treatment with nanotechnology. J. Biomed. Nanotechnol. 2018, 14, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- de Faria, C.M.; Costa, C.S.; Bagnato, V.S. Photobiomodulation effects on photodynamic therapy in HNSCC cell lines. J. Photochem. Photobiol. B Biol. 2021, 217, 112170. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar] [PubMed]
- Negri, L.B.; Martins, T.J.; da Silva, R.S.; Hamblin, M.R. Photobiomodulation combined with photodynamic therapy using ruthenium phthalocyanine complexes in A375 melanoma cells: Effects of nitric oxide generation and ATP production. J. Photochem. Photobiol. B Biol. 2019, 198, 111564. [Google Scholar] [CrossRef]
- Wang, R.; Dong, Y.; Lu, Y.; Zhang, W.; Brann, D.W.; Zhang, Q. Photobiomodulation for global cerebral ischemia: Targeting mitochondrial dynamics and functions. Mol. Neurobiol. 2019, 56, 1852–1869. [Google Scholar] [CrossRef]
- Marques, E.C.P.; Lopes, F.P.; Nascimento, I.C.; Morelli, J.; Pereira, M.V.; Meiken, V.M.M.; Pinheiro, S.L. Photobiomodulation and photodynamic therapy for the treatment of oral mucositis in patients with cancer. Photodiagn. Photodyn. Ther. 2020, 29, 101621. [Google Scholar] [CrossRef]
- Pinheiro, S.L.; Bonadiman, A.C.; Borges Lemos, A.L.d.A.; Annicchino, B.M.; Segatti, B.; Pucca, D.S.; Dutra, P.T.; de Carvalho e Silva, R.M.; Leal, F. Photobiomodulation therapy in cancer patients with mucositis: A clinical evaluation. Photobiomodul. Photomed. Laser Surg. 2019, 37, 142–150. [Google Scholar] [CrossRef]
- Tsai, S.-R.; Yin, R.; Huang, Y.-Y.; Sheu, B.-C.; Lee, S.-C.; Hamblin, M.R. Low-level light therapy potentiates NPe6-mediated photodynamic therapy in a human osteosarcoma cell line via increased ATP. Photodiagn. Photodyn. Ther. 2015, 12, 123–130. [Google Scholar] [CrossRef]
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef]
- Nelke, K.H.; Pawlak, W.; Leszczyszyn, J.; Gerber, H. Photodynamic therapy in head and neck cancer. Adv. Hyg. Exp. Med. 2014, 68, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Meulemans, J.; Delaere, P.; Vander Poorten, V. Photodynamic therapy in head and neck cancer: Indications, outcomes, and future prospects. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Chekwube, A.E.; George, B.; Abrahamse, H. Phototoxic effectiveness of zinc phthalocyanine tetrasulfonic acid on MCF-7 cells with overexpressed P-glycoprotein. J. Photochem. Photobiol. B Biol. 2020, 204, 111811. [Google Scholar] [CrossRef] [PubMed]
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. Role of Bcl-2 Family Proteins in Photodynamic Therapy Mediated Cell Survival and Regulation. Molecules 2020, 25, 5308. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef]
- Zecha, J.A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Sonis, S.T.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 1: Mechanisms of action, dosimetric, and safety considerations. Support. Care Cancer 2016, 24, 2781–2792. [Google Scholar] [CrossRef]
- Antunes, H.S.; Herchenhorn, D.; Small, I.A.; Araújo, C.M.; Viégas, C.M.P.; de Assis Ramos, G.; Dias, F.L.; Ferreira, C.G. Long-term survival of a randomized phase III trial of head and neck cancer patients receiving concurrent chemoradiation therapy with or without low-level laser therapy (LLLT) to prevent oral mucositis. Oral Oncol. 2017, 71, 11–15. [Google Scholar] [CrossRef]
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. In vitro combined effect of Doxorubicin and sulfonated zinc Phthalocyanine–mediated photodynamic therapy on MCF-7 breast cancer cells. Tumor Biol. 2017, 39, 1010428317727278. [Google Scholar] [CrossRef]
- Pevna, V.; Horvath, D.; Wagnieres, G.; Huntosova, V. Photobiomodulation and photodynamic therapy-induced switching of autophagy and apoptosis in human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2022, 234, 112539. [Google Scholar] [CrossRef]
- Jana, A.; Thomas, J.; Ghosh, P. P-glycoprotein expression in oral lichen planus. Braz. Oral Res. 2017, 31, e95. [Google Scholar] [CrossRef]
- Gameiro, M.; Silva, R.; Rocha-Pereira, C.; Carmo, H.; Carvalho, F.; Bastos, M.; Remião, F. Cellular models and in vitro assays for the screening of modulators of P-gp, MRP1 and BCRP. Molecules 2017, 22, 600. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. The P-glycoprotein multidrug transporter. Essays Biochem. 2011, 50, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.L. P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights 2013, 7, 27–34. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, W.; Zhao, J. Synergistic treatment of doxorubicin-resistant breast cancer by the combination of chemotherapy and photodynamic therapy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129167. [Google Scholar] [CrossRef]
- Jere, S.W.; Abrahamse, H.; Houreld, N.N. The JAK/STAT signaling pathway and photobiomodulation in chronic wound healing. Cytokine Growth Factor Rev. 2017, 38, 73–79. [Google Scholar] [CrossRef]
- Passarella, S.; Karu, T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J. Photochem. Photobiol. B Biol. 2014, 140, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Bensadoun, R.J.; Epstein, J.B.; Nair, R.G.; Barasch, A.; Raber-Durlacher, J.E.; Migliorati, C.; Genot-Klastersky, M.T.; Treister, N.; Arany, P.; Lodewijckx, J. Safety and efficacy of photobiomodulation therapy in oncology: A systematic review. Cancer Med. 2020, 9, 8279–8300. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.; Low, P.; McDonnell, P.A.; Laakso, E.-L.; Ralph, S.J. The effect of laser irradiation on proliferation of human breast carcinoma, melanoma, and immortalized mammary epithelial cells. Photomed. Laser Surg. 2010, 28, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Zhivotovsky, B.; Orrenius, S. Cell death mechanisms: Cross-talk and role in disease. Exp. Cell Res. 2010, 316, 1374–1383. [Google Scholar] [CrossRef]
- Tynga, I.M.; Houreld, N.; Abrahamse, H. The primary subcellular localization of Zinc phthalocyanine and its cellular impact on viability, proliferation and structure of breast cancer cells (MCF-7). J. Photochem. Photobiol. B Biol. 2013, 120, 171–176. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S. Effectiveness of Zinc-Phthalocyanine and Hypericin in Inducing Cell Death in Human Breast Cancer Cells (mcf-7) Using Low Intensity Laser Irradiation (lili); University of Johannesburg: Johannesburg, South Africa, 2013. [Google Scholar]
- Kiro, N.; Hamblin, M.; Adrahamse, H. The Effect of Low Intensity Laser Irradiation on Breast Cancer Cells and Breast Cancer Stem Cells. J. Stem Cell Res. Dev. Ther. 2019, S1005. [Google Scholar] [CrossRef]
- Wyld, L.; Reed, M.; Brown, N. Differential cell death response to photodynamic therapy is dependent on dose and cell type. Br. J. Cancer 2001, 84, 1384–1386. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Kiesslich, T.; Krammer, B.; Hammerl, P. Characterization of the cell death modes and the associated changes in cellular energy supply in response to AlPcS 4-PDT. Photochem. Photobiol. Sci. 2002, 1, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Juarranz, Á.; Gilaberte, Y.; González, S. Photodynamic therapy (PDT) in oncology. Cancers 2020, 12, 3341. [Google Scholar] [CrossRef]
- Kim, M.; Cooper, D.D.; Hayes, S.F.; Spangrude, G.J. Rhodamine-123 staining in hematopoietic stem cells of young mice indicates mitochondrial activation rather than dye efflux. Blood J. Am. Soc. Hematol. 1998, 91, 4106–4117. [Google Scholar]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Vantieghem, A.; Xu, Y.; Declercq, W.; Vandenabeele, P.; Denecker, G.; Vandenheede, J.R.; Merlevede, W.; De Witte, P.A.; Agostinis, P. Different pathways mediate cytochrome c release after photodynamic therapy with hypericin. Photochem. Photobiol. 2001, 74, 133–142. [Google Scholar] [CrossRef]






| Parameter | |
|---|---|
| Manufacturer | Optoelectronics. Tech. Co. LTD |
| Model no. | PSU—III—LED (MRL: 680–800 mW) |
| Wavelength | 681 nm |
| Wave emission | Continuous |
| Spot size | 9.1 cm2 |
| Power output | 194 ± 5 mW |
| Fluences | 20, 50, 100 J/cm2 |
| Irradiation time | 14 min ± 32 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniogo, E.C.; George, B.P.; Abrahamse, H. Photobiomodulation Improves Anti-Tumor Efficacy of Photodynamic Therapy against Resistant MCF-7 Cancer Cells. Biomedicines 2023, 11, 1547. https://doi.org/10.3390/biomedicines11061547
Aniogo EC, George BP, Abrahamse H. Photobiomodulation Improves Anti-Tumor Efficacy of Photodynamic Therapy against Resistant MCF-7 Cancer Cells. Biomedicines. 2023; 11(6):1547. https://doi.org/10.3390/biomedicines11061547
Chicago/Turabian StyleAniogo, Eric Chekwube, Blassan P. George, and Heidi Abrahamse. 2023. "Photobiomodulation Improves Anti-Tumor Efficacy of Photodynamic Therapy against Resistant MCF-7 Cancer Cells" Biomedicines 11, no. 6: 1547. https://doi.org/10.3390/biomedicines11061547