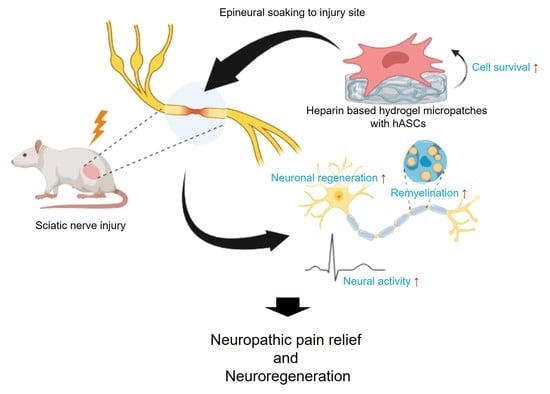
Heparin-Based Hydrogel Micropatches with Human Adipose-Derived Stem Cells: A Promising Therapeutic Approach for Neuropathic Pain Relief
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polyelectrolyte Multilayer (PEM) on ITO Electrodes by Layer-by-Layer Method
2.3. Formation of Micropatterned Heparin-Based Hydrogels on the PEM-Coated ITO Electrodes
2.4. Cell Culture and Seeding
2.5. Retrieval of Cell-Laden Micropatches by Electrochemical Stimulation
2.6. Live/Dead Staining
2.7. Neuropathic Pain Model and Implant of Micropatches
2.8. Sciatic Functional Index Analysis
2.9. Immunohistochemistry
2.10. Transmission Electron Microscopy (TEM) and Toluidine Blue Staining
2.11. Mechanical Allodynia Measurement
2.12. Electrophysiological Study
2.13. Statistical Analysis
3. Results
3.1. Heparin-Based Hydrogel Micropatches Enhance the Viability of Adherent hASCs
3.2. Implanted hASC Micropatches Restore Injured Sciatic Nerves
3.3. Transplanted hASC Micropatches Activate Remyelination in Injured Sciatic Nerves
3.4. Implanted hASC Micropatches Reduce Neuropathic Pain
3.5. Implanted hASCs Micropatches Restore Neural Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, T.S.; Baron, R.; Haanpaa, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.C.; Treede, R.D. A new definition of neuropathic pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Lee, H.L.; Yun, Y.; Kim, J.S.; Ha, Y.; Yoon, D.H.; Lee, S.H.; Shin, D.A. Human adipose stem cells improve mechanical allodynia and enhance functional recovery in a rat model of neuropathic pain. Tissue Eng. Part A 2015, 21, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Oh, J.; Yun, Y.; Lee, H.Y.; You, Y.; Che, L.; Lee, M.; Kim, K.N.; Ha, Y. Vascular endothelial growth factor-expressing neural stem cell for the treatment of neuropathic pain. Neuroreport 2015, 26, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Gilron, I.; Baron, R.; Jensen, T. Neuropathic pain: Principles of diagnosis and treatment. Mayo. Clin. Proc. 2015, 90, 532–545. [Google Scholar] [CrossRef]
- Nicholson, B.D. Diagnosis and management of neuropathic pain: A balanced approach to treatment. J. Am. Acad. Nurse Pract. 2003, 15, 3–9. [Google Scholar] [PubMed]
- Vickers, E.R.; Karsten, E.; Flood, J.; Lilischkis, R. A preliminary report on stem cell therapy for neuropathic pain in humans. J. Pain Res. 2014, 7, 255–263. [Google Scholar] [CrossRef]
- Franchi, S.; Castelli, M.; Amodeo, G.; Niada, S.; Ferrari, D.; Vescovi, A.; Brini, A.T.; Panerai, A.E.; Sacerdote, P. Adult stem cell as new advanced therapy for experimental neuropathic pain treatment. Biomed. Res. Int. 2014, 2014, 470983. [Google Scholar] [CrossRef]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-based biomaterials for peripheral nerve injury repair. Front. Bioeng. Biotechnol. 2020, 8, 554257. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Meinel, A.J.; Hilbe, M.; Meinel, L.; Merkle, H.P. Silk fibroin/hyaluronan scaffolds for human mesenchymal stem cell culture in tissue engineering. Biomaterials 2009, 30, 5068–5076. [Google Scholar] [CrossRef]
- Christopherson, G.T.; Song, H.; Mao, H.Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 2009, 30, 556–564. [Google Scholar] [CrossRef]
- Li, L.; Davidovich, A.E.; Schloss, J.M.; Chippada, U.; Schloss, R.R.; Langrana, N.A.; Yarmush, M.L. Neural lineage differentiation of embryonic stem cells within alginate microbeads. Biomaterials 2011, 32, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced bioinks for 3d printing: A materials science perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shin, Y.; Hong, B.H.; Kim, Y.J.; Chun, J.S.; Tae, G.; Kim, Y.H. In vitro chondrocyte culture in a heparin-based hydrogel for cartilage regeneration. Tissue Eng. Part C Methods 2010, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tae, G.; Kim, Y.J.; Choi, W.I.; Kim, M.; Stayton, P.S.; Hoffman, A.S. Formation of a novel heparin-based hydrogel in the presence of heparin-binding biomolecules. Biomacromolecules 2007, 8, 1979–1986. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.H.; Tae, G. Human mesenchymal stem cell culture on heparin-based hydrogels and the modulation of interactions by gel elasticity and heparin amount. Acta Biomater. 2013, 9, 7833–7844. [Google Scholar] [CrossRef]
- Gwon, K.; Kim, M.; Tae, G. A biocompatible method of controlled retrieval of cell-encapsulating microgels from a culture plate. Integr. Biol. 2014, 6, 596–602. [Google Scholar] [CrossRef]
- Gwon, K.; Kim, E.; Tae, G. Heparin-hyaluronic acid hydrogel in support of cellular activities of 3d encapsulated adipose derived stem cells. Acta Biomater. 2017, 49, 284–295. [Google Scholar] [CrossRef]
- Orthofer, M.; Valsesia, A.; Magi, R.; Wang, Q.P.; Kaczanowska, J.; Kozieradzki, I.; Leopoldi, A.; Cikes, D.; Zopf, L.M.; Tretiakov, E.O.; et al. Identification of alk in thinness. Cell 2020, 181, 1246–1262 e1222. [Google Scholar] [CrossRef]
- Arnaoutoglou, C.M.; Sakellariou, A.; Vekris, M.; Mitsionis, G.I.; Korompilias, A.; Ioakim, E.; Harhantis, A.; Beris, A. Maximum intraoperative elongation of the rat sciatic nerve with tissue expander: Functional, neurophysiological, and histological assessment. Microsurgery 2006, 26, 253–261. [Google Scholar] [CrossRef]
- de Medinaceli, L.; Freed, W.J.; Wyatt, R.J. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp. Neurol. 1982, 77, 634–643. [Google Scholar] [CrossRef]
- Petrosyan, H.A.; Alessi, V.; Sisto, S.A.; Kaufman, M.; Arvanian, V.L. Transcranial magnetic stimulation (tms) responses elicited in hindlimb muscles as an assessment of synaptic plasticity in spino-muscular circuitry after chronic spinal cord injury. Neurosci. Lett. 2017, 642, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.; Kim, Y.; Gwon, K.; Min, K.; Hwang, Y.; Tae, G. In situ formation of injectable and porous heparin-based hydrogel. Carbohydr. Polym. 2017, 174, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Chomiak, T.; Hu, B. What is the optimal value of the g-ratio for myelinated fibers in the rat cns? A theoretical approach. PLoS ONE 2009, 4, e7754. [Google Scholar] [CrossRef]
- Dehdashtian, A.; Bratley, J.V.; Svientek, S.R.; Kung, T.A.; Awan, T.M.; Cederna, P.S.; Kemp, S.W. Autologous fat grafting for nerve regeneration and neuropathic pain: Current state from bench-to-bedside. Regen. Med. 2020, 15, 2209–2228. [Google Scholar] [CrossRef]
- Miyano, K.; Ikehata, M.; Ohshima, K.; Yoshida, Y.; Nose, Y.; Yoshihara, S.I.; Oki, K.; Shiraishi, S.; Uzu, M.; Nonaka, M.; et al. Intravenous administration of human mesenchymal stem cells derived from adipose tissue and umbilical cord improves neuropathic pain via suppression of neuronal damage and anti-inflammatory actions in rats. PLoS ONE 2022, 17, e0262892. [Google Scholar] [CrossRef]
- Brini, A.T.; Amodeo, G.; Ferreira, L.M.; Milani, A.; Niada, S.; Moschetti, G.; Franchi, S.; Borsani, E.; Rodella, L.F.; Panerai, A.E.; et al. Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci. Rep. 2017, 7, 9904. [Google Scholar] [CrossRef] [PubMed]
- Simonacci, F.; Bertozzi, N.; Grieco, M.P.; Raposio, E. From liposuction to adipose-derived stem cells: Indications and technique. Acta Biomed. 2019, 90, 197–208. [Google Scholar]
- Erratum to "angiopoietin-1 modified human umbilical cord mesenchymal stem cell therapy for endotoxin-induced acute lung injury in rats" by huang zw, et al. (yonsei med j 2017 jan;58(1):206-216.). Yonsei Med. J. 2022, 63, 601. [CrossRef] [PubMed]
- Ceccarelli, S.; Pontecorvi, P.; Anastasiadou, E.; Napoli, C.; Marchese, C. Immunomodulatory effect of adipose-derived stem cells: The cutting edge of clinical application. Front. Cell Dev. Biol. 2020, 8, 236. [Google Scholar] [CrossRef]
- Zeng, N.; Chen, H.; Wu, Y.; Liu, Z. Adipose stem cell-based treatments for wound healing. Front. Cell Dev. Biol. 2021, 9, 821652. [Google Scholar] [CrossRef]
- Li, P.; Guo, X. A review: Therapeutic potential of adipose-derived stem cells in cutaneous wound healing and regeneration. Stem. Cell Res. Ther. 2018, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Johnson, T.; Liu, D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem. Cell Res. Ther. 2017, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Rautiainen, S.; Laaksonen, T.; Koivuniemi, R. Angiogenic effects and crosstalk of adipose-derived mesenchymal stem/stromal cells and their extracellular vesicles with endothelial cells. Int. J. Mol. Sci. 2021, 22, 10890. [Google Scholar] [CrossRef]
- Jankowski, M.; Dompe, C.; Sibiak, R.; Wasiatycz, G.; Mozdziak, P.; Jaskowski, J.M.; Antosik, P.; Kempisty, B.; Dyszkiewicz-Konwinska, M. In vitro cultures of adipose-derived stem cells: An overview of methods, molecular analyses, and clinical applications. Cells 2020, 9, 1783. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, W.; Rubin, J.P.; Marra, K.G. Adipose-derived stem cells: Implications in tissue regeneration. World J. Stem. Cells 2014, 6, 312–321. [Google Scholar] [CrossRef]
- Kang, N.U.; Lee, S.J.; Gwak, S.J. Fabrication techniques of nerve guidance conduits for nerve regeneration. Yonsei Med. J. 2022, 63, 114–123. [Google Scholar] [CrossRef]
- Prautsch, K.M.; Schmidt, A.; Paradiso, V.; Schaefer, D.J.; Guzman, R.; Kalbermatten, D.F.; Madduri, S. Modulation of human adipose stem cells’ neurotrophic capacity using a variety of growth factors for neural tissue engineering applications: Axonal growth, transcriptional, and phosphoproteomic analyses in vitro. Cells 2020, 9, 1939. [Google Scholar] [CrossRef]
- Ribitsch, I.; Oreff, G.L.; Jenner, F. Regenerative medicine for equine musculoskeletal diseases. Animals 2021, 11, 234. [Google Scholar] [CrossRef]
- Kocsis, J.D.; Lankford, K.L.; Sasaki, M.; Radtke, C. Unique in vivo properties of olfactory ensheathing cells that may contribute to neural repair and protection following spinal cord injury. Neurosci. Lett. 2009, 456, 137–142. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Z.; Smith, G.M.; Wen, X.; Pressman, Y.; Wood, P.M.; Xu, X.M. Gdnf-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia 2009, 57, 1178–1191. [Google Scholar] [CrossRef]
- Salio, C.; Ferrini, F. Bdnf and gdnf expression in discrete populations of nociceptors. Ann. Anat. 2016, 207, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wong, A.W.; Willingham, M.M.; Kaasinen, S.K.; Hendry, I.A.; Howitt, J.; Putz, U.; Barrett, G.L.; Kilpatrick, T.J.; Murray, S.S. Bdnf exerts contrasting effects on peripheral myelination of ngf-dependent and bdnf-dependent drg neurons. J. Neurosci. 2009, 29, 4016–4022. [Google Scholar] [CrossRef] [PubMed]
- Yun, W.; Choi, K.A.; Hwang, I.; Zheng, J.; Park, M.; Hong, W.; Jang, A.Y.; Kim, J.H.; Choi, W.; Kim, D.S.; et al. Oct4-induced oligodendrocyte progenitor cells promote remyelination and ameliorate disease. NPJ Regen. Med. 2022, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Liu, Y.; Xiao, F.; Zhang, L.; Li, W.; Wang, B.; Weng, Z.; Liu, Y.; Chen, G. Research progress of hydrogels as delivery systems and scaffolds in the treatment of secondary spinal cord injury. Front. Bioeng. Biotechnol. 2023, 11, 1111882. [Google Scholar] [CrossRef]
- Lv, Z.; Dong, C.; Zhang, T.; Zhang, S. Hydrogels in spinal cord injury repair: A review. Front. Bioeng. Biotechnol. 2022, 10, 931800. [Google Scholar] [CrossRef]
- Qiu, C.; Sun, Y.; Li, J.; Xu, Y.; Zhou, J.; Qiu, C.; Zhang, S.; He, Y.; Yu, L. Therapeutic effect of biomimetic scaffold loaded with human amniotic epithelial cell-derived neural-like cells for spinal cord injury. Bioengineering 2022, 9, 535. [Google Scholar] [CrossRef]
- Holdefer, R.N.; MacDonald, D.B.; Skinner, S.A. Somatosensory and motor evoked potentials as biomarkers for post-operative neurological status. Clin. Neurophysiol. 2015, 126, 857–865. [Google Scholar] [CrossRef]
- Haghighi, S.S.; York, D.H.; Gaines, R.W.; Oro, J.J. Monitoring of motor tracts with spinal cord stimulation. Spine 1994, 19, 1518–1524. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Tae, G.; Hwang, S.; Wee, S.; Ha, Y.; Lee, H.-L.; Shin, D. Heparin-Based Hydrogel Micropatches with Human Adipose-Derived Stem Cells: A Promising Therapeutic Approach for Neuropathic Pain Relief. Biomedicines 2023, 11, 1436. https://doi.org/10.3390/biomedicines11051436
Lee H, Tae G, Hwang S, Wee S, Ha Y, Lee H-L, Shin D. Heparin-Based Hydrogel Micropatches with Human Adipose-Derived Stem Cells: A Promising Therapeutic Approach for Neuropathic Pain Relief. Biomedicines. 2023; 11(5):1436. https://doi.org/10.3390/biomedicines11051436
Chicago/Turabian StyleLee, HyeYeong, GiYoong Tae, SaeYeon Hwang, SungWon Wee, Yoon Ha, Hye-Lan Lee, and DongAh Shin. 2023. "Heparin-Based Hydrogel Micropatches with Human Adipose-Derived Stem Cells: A Promising Therapeutic Approach for Neuropathic Pain Relief" Biomedicines 11, no. 5: 1436. https://doi.org/10.3390/biomedicines11051436