Ameliorative Effects of Isoeugenol and Eugenol against Impaired Nerve Function and Inflammatory and Oxidative Mediators in Diabetic Neuropathic Rats
Abstract
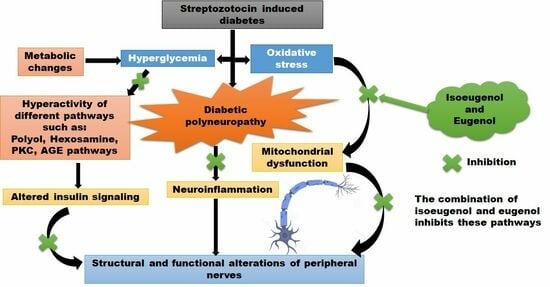
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Experimental Design
2.3.1. Selection of Doses and Grouping of Animals
2.3.2. Induction of Diabetic Neuropathy
2.3.3. Body Weight, Food Consumption, and Water Intake
2.3.4. Estimation of Antioxidant Parameters
2.3.5. Estimation of TNF-α and NGF Expressions
2.3.6. Histopathology
2.4. Behavioral Studies
2.4.1. Determination of Paw Cold Allodynia (Acetone Sprinkling Test)
2.4.2. Determination of Mechanical Hyperalgesia (Pinprick Test)
2.4.3. Determination of Paw Heat Hyperalgesia (Hot-Plate Test)
2.4.4. Determination of Mechanical Allodynia (Von Frey Hair Test)
2.5. Statistical Analysis
3. Results
3.1. Effect of Isoeugenol and Eugenol on Change in Body Weight of Experimental Rats
3.2. Effect of Isoeugenol and Eugenol on Blood Glucose Level
3.3. Effect of Isoeugenol and Eugenol on Food and Water Consumption
3.4. Effect of Isoeugenol and Eugenol on Antioxidant Parameters
3.5. Effect of Isoeugenol and Eugenol on TNF-α
3.6. Effect of Isoeugenol and Eugenol on NGF
3.7. Effect of Isoeugenol and Eugenol on Hyperalgesia and Allodynia
3.8. Histopathology Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kajal, A.; Singh, R. Coriandrum sativum improve neuronal function via inhibition of oxidative/nitrosative stress and TNF-α in diabetic neuropathic rats. J. Ethnopharmacol. 2020, 263, 112959. [Google Scholar] [CrossRef] [PubMed]
- Sinnreich, M.; Taylor, B.V.; Dyck, P.J.B. Diabetic neuropathies: Classification, clinical features, and pathophysiological basis. Neurologist 2005, 11, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Wada, R.; Yagihashi, S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann. N. Y. Acad. Sci. 2005, 1043, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Kaur, S.; Pandhi, P.; Dutta, P. Painful diabetic neuropathy: An update. Ann. Neurosci. 2011, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Hellweg, R.; Hartung, H.-D. Endogenous levels of nerve growth factor (NGF) are altered in experimental diabetes mellitus: A possible role for NGF in the pathogenesis of diabetic neuropathy. J. Neurosci. Res. 1990, 26, 258–267. [Google Scholar] [CrossRef]
- Jiang, W.J.; Peng, Y.C.; Yang, K.M. Cellular signaling pathways regulating β-cell proliferation as a promising therapeutic target in the treatment of diabetes. Exp. Ther. Med. 2018, 16, 3275–3285. [Google Scholar] [CrossRef] [Green Version]
- Sima, A.A.F. New insights into the metabolic and molecular basis for diabetic neuropathy. Cell. Mol. Life Sci. 2003, 60, 2445–2464. [Google Scholar] [CrossRef]
- Sima, A.A.; Calvani, M.; Mehra, M.; Amato, A.; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: An analysis of two randomized placebo-controlled trials. Diabetes Care 2005, 28, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Demir, S.; Nawroth, P.P.; Herzig, S.; Üstünel, B.E. Emerging targets in type 2 diabetes and diabetic complications. Adv. Sci. 2021, 8, 2100275. [Google Scholar] [CrossRef]
- Hanaoka, Y.; Ohi, T.; Furukawa, S.; Furukawa, Y.; Hayashi, K.; Matsukura, S. The therapeutic effects of 4-methylcatechol, a stimulator of endogenous nerve growth factor syn-thesis, on experimental diabetic neuropathy in rats. J. Neurol. Sci. 1994, 122, 28–32. [Google Scholar] [CrossRef]
- Ito, S.; Pham, V.M.; Matsumura, S.; Katano, T.; Funatsu, N. Diabetic neuropathy research: From mouse models to targets for treatment. Neural Regen. Res. 2019, 14, 1870–1879. [Google Scholar] [CrossRef]
- Kim, N.; Kim, S.H.; Kim, Y.J.; Kim, J.K.; Nam, M.K.; Rhim, H.; Yoon, S.K.; Choi, S.-Z.; Son, M.; Kim, S.-Y.; et al. Neurotrophic activity of DA-9801, a mixture extract of Dioscorea japonica Thunb. and Dioscorea nipponica Makino, in vitro. J. Ethnopharmacol. 2011, 137, 312–319. [Google Scholar] [CrossRef]
- Choi, J.G.; Khan, Z.; Choi, S.-Z.; Kim, S.Y.; Oh, M.S. DA-9801, a standardized Dioscorea extract, improves memory function via the activation of nerve growth factor-mediated signaling. Nutr. Neurosci. 2022, 25, 219–230. [Google Scholar] [CrossRef]
- Hu, D.-R. Effect of mouse nerve growth factor combined with mecobalamine on treatment of diabetic peripheral neuropathy. J. Hainan Med. Univ. 2016, 22, 51–54. [Google Scholar]
- Khan, N.; Smith, M.T. Neurotrophins and neuropathic pain: Role in pathobiology. Molecules 2015, 20, 10657–10688. [Google Scholar] [CrossRef] [Green Version]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A natural compound with versatile pharmacological actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef] [Green Version]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological properties and health benefits of eugenol: A comprehensive review. Oxidative Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Fujisawa, S.; Murakami, Y. Eugenol and its role in chronic diseases. In Drug Discovery from Mother Nature; Springer: Berlin/Heidelberg, Germany, 2016; Volume 929, pp. 45–66. [Google Scholar] [CrossRef]
- Rajakumar, D.; Rao, M. Dehydrozingerone and isoeugenol as inhibitors of lipid peroxidation and as free radical scavengers. Biochem. Pharmacol. 1993, 46, 2067–2072. [Google Scholar] [CrossRef]
- Choi, C.Y.; Park, K.R.; Lee, J.H.; Jeon, Y.J.; Liu, K.H.; Oh, S.; Kim, D.-E.; Yea, S.S. Isoeugenol suppression of inducible nitric oxide synthase expression is mediated by down-regulation of NF-κB, ERK1/2, and p38 kinase. Eur. J. Pharmacol. 2007, 576, 151–159. [Google Scholar] [CrossRef]
- Pavela, R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zheng, Z. Animal models of diabetic neuropathic pain. Exp. Clin. Endocrinol. Diabetes 2014, 122, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonawane, R.D.; Vishwakarma, S.L.; Lakshmi, S.; Rajani, M.; Padh, H.; Goyal, R.K. Amelioration of STZ-induced type 1 diabetic nephropathy by aqueous extract of Enicostemma littorale Blume and swertiamarin in rats. Mol. Cell. Biochem. 2010, 340, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Bansal, M.K.; Dalvi, R.; Upganlawar, A.; Somani, R. Protective effect of diosmin against diabetic neuropathy in experimental rats. J. Integr. Med. 2014, 12, 35–41. [Google Scholar] [CrossRef]
- Kumar, A.; Kaundal, R.K.; Iyer, S.; Sharma, S.S. Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sci. 2007, 80, 1236–1244. [Google Scholar] [CrossRef]
- Kaur, N.; Kishore, L.; Singh, R. Chromane isolated from leaves of Dillenia indica improves the neuronal dysfunction in STZ-induced diabetic neuropathy. J. Ethnopharmacol. 2017, 206, 19–30. [Google Scholar] [CrossRef]
- Salbitani, G.; Bottone, C.; Carfagna, S. Determination of reduced and total glutathione content in extremophilic microalga galdieria phlegrea. Bio-Protocol 2017, 7, e2372. [Google Scholar] [CrossRef] [Green Version]
- Sah, D.K.; Nagarathana, P.K. Screening of cardioprotective activity of leaves of Andrographis paniculata against isoproterenol induced myocardial infarction in rats. Int. J. Pharmacol. Res. 2016, 6, 23–28. [Google Scholar]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of superoxide dismutase (SOD). In Plant-Microbe Interactions; Humana: New York, NY, USA, 2021; pp. 117–118. [Google Scholar]
- Flatters, S.J.; Bennett, G.J. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain 2004, 109, 150–161. [Google Scholar] [CrossRef]
- Erichsen, H.K.; Blackburn-Munro, G. Pharmacological characterisation of the spared nerve injury model of neuropathic pain. Pain 2002, 98, 151–161. [Google Scholar] [CrossRef]
- Foudah, A.I.; Alqarni, M.H.; Devi, S.; Singh, A.; Alam, A.; Alam, P.; Singh, S. Analgesic Action of Catechin on Chronic Constriction Injury–Induced Neuropathic Pain in Sprague–Dawley Rats. Front. Pharmacol. 2022, 13, 895079. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- O’Connor, A.B.; Dworkin, R.H. Treatment of neuropathic pain: An overview of recent guidelines. Am. J. Med. 2009, 122, S22–S32. [Google Scholar] [CrossRef]
- Tesfaye, S. Neuropathy in diabetes. Medicine 2015, 43, 26–32. [Google Scholar] [CrossRef]
- Vinik, A.; Ullal, J.; Parson, H.K.; Casellini, C.M. Diabetic neuropathies: Clinical manifestations and current treatment options. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 269–281. [Google Scholar] [CrossRef]
- Watson, J.J.; Allen, S.J.; Dawbarn, D. Targeting Nerve Growth Factor in Pain. Biodrugs 2008, 22, 349–359. [Google Scholar] [CrossRef]
- Sommer, C.; Kress, M. Recent findings on how pro-inflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 2004, 361, 184–187. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Pacheco-Moisés, F.P.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G. The role of oxidative stress, mitochondrial function, and autophagy in diabetic polyneuropathy. J. Diabetes Res. 2017, 2017, 1673081. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.-H.; Seog, H.-M.; Choi, I.-W.; Choi, H.-D.; Cho, H.-Y. Effects of wild ginseng (Panax ginseng C.A. Meyer) leaves on lipid peroxidation levels and antioxidant enzyme activities in streptozotocin diabetic rats. J. Ethnopharmacol. 2005, 98, 245–250. [Google Scholar] [CrossRef]
- Wadhwa, K.; Pahwa, R.; Kumar, M.; Kumar, S.; Sharma, P.C.; Singh, G.; Verma, R.; Mittal, V.; Singh, I.; Kaushik, D.; et al. Mechanistic insights into the pharmacological significance of silymarin. Molecules 2022, 27, 5327. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, T.G.; Garg, N.; Dhiman, S.; Gupta, S.; Rahman, H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Dysbiosis and alzheimer’s disease: A role for chronic stress? Biomolecules 2021, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- King, G.L. The role of inflammatory cytokines in diabetes and its complications. J. Periodontol. 2008, 79, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Wild, K.D.; Bian, D.; Zhu, D.; Davis, J.; Bannon, A.W.; Zhang, T.J.; Louis, J.-C. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. Experiment 2007, 322, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Naseri, R.; Farzaei, F.; Fakhri, S.; El-Senduny, F.F.; Altouhamy, M.; Bahramsoltani, R.; Ebrahimi, F.; Rahimi, R.; Farzaei, M.H. Polyphenols for diabetes associated neuropathy: Pharmacological targets and clinical perspective. DARU J. Pharm. Sci. 2019, 27, 781–798. [Google Scholar] [CrossRef]
- Kandhare, A.D.; Raygude, K.S.; Kumar, V.S.; Rajmane, A.R.; Visnagri, A.; Ghule, A.E.; Ghosh, P.; Badole, S.L.; Bodhankar, S.L. Ameliorative effects quercetin against impaired motor nerve function, inflammatory mediators and apoptosis in neonatal streptozotocin-induced diabetic neuropathy in rats. Biomed. Aging Pathol. 2012, 2, 173–186. [Google Scholar] [CrossRef]
- Bagdas, D.; Ozboluk, H.Y.; Cinkilic, N.; Gurun, M.S. Antinociceptive effect of chlorogenic acid in rats with painful diabetic neu-ropathy. J. Med. Food 2014, 17, 730–732. [Google Scholar] [CrossRef]
- Akbar, S.; Subhan, F.; Karim, N.; Shahid, M.; Ahmad, N.; Ali, G.; Mahmood, W.; Fawad, K. 6-Methoxyflavanone attenuates mechanical allodynia and vulvodynia in the streptozotocin-induced diabetic neuropathic pain. Biomed. Pharmacother. 2016, 84, 962–971. [Google Scholar] [CrossRef]
- Attia, H.N.; Al-Rasheed, N.M.; Al-Rasheed, N.M.; Maklad, Y.A.; Ahmed, A.A.E.; Kenawy, S.A.B. Protective effects of combined therapy of gliclazide with curcumin in experimental diabetic neuropathy in rats. Behav. Pharmacol. 2012, 23, 153–161. [Google Scholar] [CrossRef]
- Abd Allah, E.S.; Gomaa, A.M. Effects of curcumin and captopril on the functions of kidney and nerve in streptozotocin-induced diabetic rats: Role of angiotensin converting enzyme 1. Appl. Physiol. Nutr. Metab. 2015, 40, 1061–1067. [Google Scholar] [CrossRef]
- Nagilla, B.; Pratap, R.K. Neuroprotective and antinociceptive effect of curcumin in diabetic neuropathy in rats. Int. J. Pharm. Pharm. Sci. 2014, 6, 131–138. [Google Scholar]
- Ghatak, S.B.; Panchal, S.S. Protective effect of oryzanol isolated from crude rice bran oil in experimental model of diabetic neu-ropathy. Rev. Bras. Farmacogn. 2012, 22, 1092–1103. [Google Scholar] [CrossRef] [Green Version]
- Baluchnejadmojarad, T.; Roghani, M.; Mafakheri, M. Neuroprotective effect of silymarin in 6-hydroxydopamine hemi-parkinsonian rat: Involvement of estrogen receptors and oxidative stress. Neurosci. Lett. 2010, 480, 206–210. [Google Scholar] [CrossRef]
- Rocha-González, H.I.; Ramírez-Aguilar, M.; Granados-Soto, V.; Reyes-García, J.G.; Torres-López, J.E.; Huerta-Cruz, J.C.; Navarrete, A. Antineuropathic effect of 7-hydroxy-3,4-dihydrocadalin in streptozotocin-induced diabetic rodents. BMC Complement. Altern. Med. 2014, 14, 129. [Google Scholar] [CrossRef]
- AlSharari, S.D.; Al-Rejaie, S.S.; Abuohashish, H.M.; Aleisa, A.M.; Parmar, M.Y.; Ahmed, M.M. Ameliorative potential of morin in strep-tozotocin-induced neuropathic pain in rats. Trop. J. Pharm. Res. 2014, 13, 1429–1436. [Google Scholar] [CrossRef] [Green Version]
- Marco, F.; Aashay, P.; Sohum, S.; Akshay, R.; Brandon, L.-W.; Foreman, M.; Patel, A.; Sheth, S.; Reddy, A.; Lucke-Wold, B.; et al. Diabetes Mellitus Management in the Context of Cranial Tumors. BOHR Int. J. Neurol. Neurosci. 2022, 1, 29–39. [Google Scholar] [CrossRef]
- Michel, M.; Lucke-Wold, B. Diabetes management in spinal surgery. J. Clin. Images Med. Case Rep. 2022, 3, 1906. [Google Scholar]
- Grover, M.; Shah, K.; Khullar, G.; Gupta, J.; Behl, T. Investigation of the utility of Curcuma caesia in the treatment of diabetic neuropathy. J. Pharm. Pharmacol. 2019, 71, 725–732. [Google Scholar] [CrossRef] [Green Version]








| Group (s) | % Body Weight Variation |
|---|---|
| Normal control | 12.64 ± 2.61 |
| Diabetic control | −20.80± 2.26 ***,a |
| STZ + pregabalin | 10.38 ± 3.40 **,b |
| STZ + isoeugenol | 11.04 ± 6.71 **,b |
| STZ + eugenol | 11.67 ± 21.74 **,b |
| STZ + isoeugenol + eugenol | 16.04 ± 6.71 ***,b |
| STZ + anti-NGF | 6.04 ± 6.71 *,b |
| Groups (45th Day) | TBARS (mmole/mg of Protein) | SOD (units/mg of Protein) | Catalase (units/mg of Protein) | GSH (mmole/mg of Protein) |
|---|---|---|---|---|
| Normal control | 1.12 ± 0.05 | 3.03 ± 0.05 | 3.78 ± 0.05 | 2.66 ± 0.16 |
| Diabetic control | 2.85 ± 0.22 *,a | 1.04 ± 0.08 *,a | 0.56 ± 0.02 *,a | 0.79 ± 0.10 *,a |
| STZ + pregabalin | 1.88 ± 0.11 *,b | 2.44 ± 0.07 *,b | 3.19 ± 0.02 *,b | 2.14 ± 0.06 *,b |
| STZ + isoeugenol | 2.22 ± 0.09 | 2.22 ± 0.04 *,b | 2.18 ± 0.05 *,b | 1.94 ± 0.14 *,b |
| STZ + eugenol | 1.89 ± 0.07 **,b | 2.55 ± 0.01 *,b | 2.71 ± 0.03 *,b | 2.08 ± 0.05 *,b |
| STZ + isoeugenol + eugenol | 1.69 ± 0.05 *,b | 2.93 ± 0.05 *,b | 3.08 ± 0.05 *,b | 2.36 ± 0.16 *,b |
| STZ + anti-NGF | 2.45 ± 0.22 | 0.84 ± 0.08 | 1.16 ± 0.02 | 1.14 ± 0.10 |
| Group (45th Day) | Nerve Degeneration Score |
|---|---|
| Normal control | 0.02 ± 0.01 |
| Diabetic control | 2.89 ± 0.31 *,a |
| STZ + pregabalin | 0.98 ± 0.11 *,b |
| STZ + isoeugenol | 2.32 ± 0.29 |
| STZ + eugenol | 1.89 ± 0.27 |
| STZ + isoeugenol + eugenol | 1.69 ± 0.25 *,b |
| STZ + anti-NGF | 2.15 ± 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthy, K.M.; Balaha, M.F.; Devi, S.; Altharawi, A.; Yusufoglu, H.S.; Aldossari, R.M.; Alam, A.; di Giacomo, V. Ameliorative Effects of Isoeugenol and Eugenol against Impaired Nerve Function and Inflammatory and Oxidative Mediators in Diabetic Neuropathic Rats. Biomedicines 2023, 11, 1203. https://doi.org/10.3390/biomedicines11041203
Alharthy KM, Balaha MF, Devi S, Altharawi A, Yusufoglu HS, Aldossari RM, Alam A, di Giacomo V. Ameliorative Effects of Isoeugenol and Eugenol against Impaired Nerve Function and Inflammatory and Oxidative Mediators in Diabetic Neuropathic Rats. Biomedicines. 2023; 11(4):1203. https://doi.org/10.3390/biomedicines11041203
Chicago/Turabian StyleAlharthy, Khalid M., Mohamed F. Balaha, Sushma Devi, Ali Altharawi, Hasan S. Yusufoglu, Rana M. Aldossari, Aftab Alam, and Viviana di Giacomo. 2023. "Ameliorative Effects of Isoeugenol and Eugenol against Impaired Nerve Function and Inflammatory and Oxidative Mediators in Diabetic Neuropathic Rats" Biomedicines 11, no. 4: 1203. https://doi.org/10.3390/biomedicines11041203