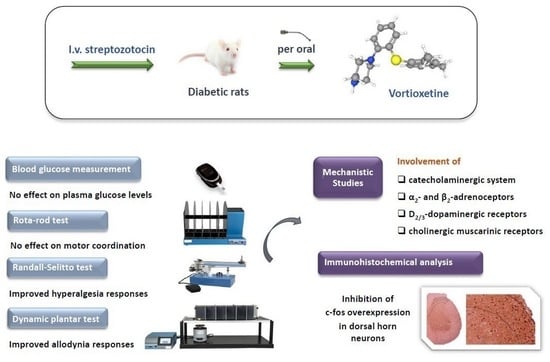
Catecholaminergic and Cholinergic Systems Mediate Beneficial Effect of Vortioxetine on Diabetes-Induced Neuropathic Pain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs
2.2. Animals
2.3. Induction of Diabetes Model on Animals
2.4. Pharmacological Treatment Protocol
2.5. Measurement of Plasma Glucose Levels
2.6. Motor Coordination Experiments
2.7. Neuropathic Pain Experiments
2.7.1. Randall–Selitto Test
2.7.2. Dynamic Plantar Test
2.8. Studies for the Underlying Mechanisms
2.9. Immunohistochemical Analyses
2.9.1. Histopathological Procedure
2.9.2. Immunohistochemical Staining
2.9.3. Microscopy and Immunohistochemistry
2.10. Statistical Evaluation
3. Results
3.1. Effects of Vortioxetine Treatment on Blood Glucose Levels in Diabetic Rats
3.2. Effects of Vortioxetine Treatment on Motor Coordination of Diabetic Rats
3.3. Effects of Vortioxetine on Diabetes-Induced Neuropathic Pain
3.3.1. Randall–Selitto Test Results
3.3.2. Dynamic Plantar Test Results
3.4. Mechanistic Studies
3.4.1. Participation of Catecholaminergic System in the Beneficial Effect of Vortioxetine on Diabetes-Induced Mechanical Hyperalgesia and Allodynia
3.4.2. Participation of Cholinergic System in the Beneficial Effect of Vortioxetine on Diabetes-Induced Mechanical Hyperalgesia and Allodynia
3.5. Vortioxetine-Induced c-Fos Immunoreactivity in the Dorsal Horn of Diabetic Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frampton, J.E. Vortioxetine: A Review in Cognitive Dysfunction in Depression. Drugs 2019, 76, 1675–1682. [Google Scholar] [CrossRef]
- Gonda, X.; Sharma, S.R.; Tarazi, F.I. Vortioxetine: A novel antidepressant for the treatment of major depressive disorder. Expert. Opin. Drug Discov. 2019, 14, 81–89. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Brintellix: Assessment Report as Adopted by the Committee for Medicinal Products for Human Use (CHMP); EMA: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Dziwota, E.; Olajossy, M. Vortioxetine-the new antidepressant agent with procognitive properties. Acta Pol. Pharm. 2016, 73, 1433–1437. [Google Scholar] [PubMed]
- Du Jardin, K.G.; Liebenberg, N.; Müller, H.K.; Elfving, B.; Sanchez, C.; Wegener, G. Differential interaction with the serotonin system by S-ketamine, vortioxetine, and fluoxetine in a genetic rat model of depression. Psychopharmacology 2016, 233, 2813–2825. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.M.; Fixen, D.R.; Linnebur, S.A.; Pearson, S.M. Cognitive effects of vortioxetine in older adults: A systematic review. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211026796. [Google Scholar] [CrossRef] [PubMed]
- Adamo, D.; Calabria, E.; Coppola, N.; Pecoraro, G.; Mignogna, M.D. Vortioxetine as a new frontier in the treatment of chronic neuropathic pain: A review and update. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211034320. [Google Scholar] [CrossRef]
- Pehrson, A.L.; Cremers, T.; Betry, C.; van der Hart, M.G.; Jorgensen, L.; Madsen, M.; Haddjeri, N.; Ebert, B.; Sanchez, C. Lu AA21004, a novel multimodal antidepressant, produces regionally selective increases of multiple neurotransmitters—A rat microdialysis and electrophysiology study. Eur. Neuropsychopharmacol. 2013, 23, 133–145. [Google Scholar] [CrossRef]
- Fernandez-Pastor, B.; Ortega, J.E.; Meana, J.J. Involvement of serotonin 5-HT3 receptors in the modulation of noradrenergic transmission by serotonin reuptake inhibitors: A microdialysis study in rat brain. Psychopharmacology 2013, 229, 331–344. [Google Scholar] [CrossRef]
- Diaz-Mataix, L.; Scorza, M.C.; Bortolozzi, A.; Toth, M.; Celada, P.; Artigas, F. Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity:role in atypical antipsychotic action. J. Neurosci. 2005, 25, 10831–10843. [Google Scholar] [CrossRef] [Green Version]
- Todorović, M.; Micov, A.; Nastić, K.; Tomić, M.; Pecikoza, U.; Vuković, M.; Stepanović-Petrović, R. Vortioxetine as an analgesic in preclinical inflammatory pain models: Mechanism of action. Fundam. Clin. Pharmacol. 2022, 36, 237–249. [Google Scholar] [CrossRef]
- Turan Yücel, N.; Kandemir, Ü.; Demir Özkay, Ü.; Can, Ö.D. 5-HT1A Serotonergic, α-adrenergic and opioidergic receptors mediate the analgesic efficacy of vortioxetine in mice. Molecules 2021, 26, 3242. [Google Scholar] [CrossRef] [PubMed]
- Inaltekin, A.; Kivrak, Y. Evaluation of the Effect of Vortioxetine on Pain Threshold by Hot-Plate Test in Mice. Noro Psikiyatr Ars. 2021, 58, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Alcántara Montero, A.; de Vasconcelos, S.R.P. Role of vortioxetine in the treatment of neuropathic pain. Rev. Esp. Anestesiol. Reanim. Engl. Ed. 2022, 69, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Sałat, K.; Furgała-Wojas, A. Serotonergic neurotransmission system modulator, vortioxetine, and dopaminergic d2/d3 receptor agonist, ropinirole, attenuate fibromyalgia-like symptoms in mice. Molecules 2021, 26, 2398. [Google Scholar] [CrossRef]
- Zuena, A.R.; Maftei, D.; Alemà, G.S.; Dal Moro, F.; Lattanzi, R.; Casolini, P.; Nicoletti, F. Multimodal antidepressant vortioxetine causes analgesia in a mouse model of chronic neuropathic pain. Mol. Pain 2018, 14, 1744806918808987. [Google Scholar] [CrossRef] [Green Version]
- Adamo, D.; Pecoraro, G.; Aria, M.; Favia, G.; Mignogna, M.D. Vortioxetine in the Treatment of Mood Disorders Associated with Burning Mouth Syndrome: Results of an Open-Label, Flexible-Dose Pilot Study. Pain Med. 2020, 21, 185–194. [Google Scholar] [CrossRef]
- Micov, A.M.; Tomić, M.A.; Todorović, M.B.; Vuković, M.J.; Pecikoza, U.B.; Jasnic, N.I.; Djordjevic, J.D.; Stepanović-Petrović, R.M. Vortioxetine reduces pain hypersensitivity and associated depression-like behavior in mice with oxaliplatin-induced neuropathy. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 103, 109975. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albers, J.W. Diabetic neuropathy: Mechanisms, emerging treatments and subtypes. Curr. Neurol. Neurosci. Rep. 2014, 14, 473. [Google Scholar] [CrossRef] [Green Version]
- Schäffler, A. ASP0160 Is diabetes an inflammatory disease and should be treated like that? Ann. Rheum. Dis. 2017, 76, 39. [Google Scholar]
- Attal, N.; Cruccu, G.; Baron, R.; Haanpää, M.; Hansson, P.; Jensen, T.S.; Nurmikko, T. European Federation of Neurological Societies (2010) EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur. J. Neurol. 2010, 17, 1113-e88. [Google Scholar] [CrossRef] [PubMed]
- Pehrson, A.L.; Hillhouse, T.M.; Haddjeri, N.; Rovera, R.; Porter, J.H.; Mørk, A.; Smagin, G.; Song, D.; Budac, D.; Cajina, M.; et al. Task- and treatment length-dependent effects of vortioxetine on scopolamine-induced cognitive dysfunction and hippocampal extracellular acetylcholine in rats. J. Pharmacol. Exp. Ther. 2016, 358, 472–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa-Méndez, S.; Perez-Sánchez, G.; Salazar-Juárez, A. Vortioxetine treatment decreases cocaine-induced locomotor sensitization in rats. Physiol. Behav. 2022, 257, 113989. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Üçel, U.İ.; Can, Ö.D.; Demir Özkay, Ü.; Öztürk, Y. Antihyperalgesic and antiallodynic effects of mianserin on diabetic neuropathic pain: A study on mechanism of action. Eur. J. Pharmacol. 2015, 756, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Barbaros, M.B.; Can, Ö.D.; Üçel, U.İ.; Turan Yücel, N.; Demir Özkay, Ü. Antihyperalgesic activity of atomoxetine on diabetes-induced neuropathic pain: Contribution of noradrenergic and dopaminergic systems. Molecules 2018, 23, 2072. [Google Scholar] [CrossRef] [Green Version]
- Can, O.D.; Oztürk, Y.; Ozkay, U.D. Effects of insulin and St. John’s Wort treatments on anxiety, locomotory activity, depression, and active learning parameters of streptozotocin-diabetic rats. Planta Med. 2011, 77, 1970–1976. [Google Scholar] [CrossRef]
- Bétry, C.; Etiévant, A.; Pehrson, A.; Sánchez, C.; Haddjeri, N. Effect of the multimodal acting antidepressant vortioxetine on rat hippocampal plasticity and recognition memory. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 58, 38–46. [Google Scholar] [CrossRef]
- Ozbeyli, D.; Aykac, A.; Alaca, N.; Hazar-Yavuz, A.N.; Ozkan, N.; Sener, G. Protective effects of vortioxetine in predator scent stress model of post-traumatic stress disorder in rats: Role on neuroplasticity and apoptosis. J. Physiol. Pharmacol. 2019, 70, 557–571. [Google Scholar]
- Spolidório, P.C.; Echeverry, M.B.; Iyomasa, M.; Guimarães, F.S.; Del Bel, E.A. Anxiolytic effects induced by inhibition of the nitric oxide-cGMP pathway in the rat dorsal hipocampus. Psychopharmacology 2007, 195, 183–192. [Google Scholar] [CrossRef]
- Bordet, T.; Buisson, B.; Michaud, M.; Abitbol, J.L.; Marchand, F.; Grist, J.; Andriambeloson, E.; Malcangio, M.; Pruss, R.M. Specific antinociceptive activity of cholest-4-en-3-one, oxime (TRO19622) in experimental models of painful diabetic and chemotherapy-induced neuropathy. J. Pharmacol. Exp. Ther. 2008, 326, 623–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydın, T.H.; Can, Ö.D.; Demir Özkay, Ü.; Turan, N. Effect of subacute agomelatine treatment on painful diabetic neuropathy: Involvement of catecholaminergic mechanisms. Fundam. Clin. Pharmacol. 2016, 30, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Xue, R.; Zhu, L.; Li, J.; Fan, Q.Y.; Zhong, B.H.; Li, Y.F.; Ye, C.Y.; Zhang, Y.Z. Evaluation of the analgesic effects of ammoxetine, a novel potent serotonin and norepinephrine reuptake inhibitor. Acta Pharmacol. Sin. 2016, 37, 1154–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nogueira-Neto, F.S.; Amorim, R.L.; Brigatte, P.; Picolo, G.; Ferreira, W.A., Jr.; Gutierrez, V.P.; Conceição, I.M.; Della-Casa, M.S.; Takahira, R.K.; Nicoletti, J.L.; et al. The analgesic effect of crotoxin on neuropathic pain is mediated by central muscarinic receptors and 5-lipoxygenase-derived mediators. Pharmacol. Biochem. Behav. 2008, 91, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Feng, X.; Dong, R.; Wang, H.; Liu, J.; Li, W.; Xu, J.; Yu, B. Effects of clonidine on bilateral pain behaviors and inflammatory response in rats under the state of neuropathic pain. Neurosci. Lett. 2011, 505, 254–259. [Google Scholar] [CrossRef]
- Njung’e, K.; Critchley, M.A.; Handley, S.L. Effects of beta-adrenoceptor ligands in the elevated X-maze ‘anxiety’ model and antagonism of the ’anxiogenic’ response to 8-OH-DPAT. J. Psychopharmacol. 1993, 7, 173–180. [Google Scholar] [CrossRef]
- Forman, L.J. NMDA receptor antagonism produces antinociception which is partially mediated by brain opioids and dopamine. Life Sci. 1999, 64, 1877–1887. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Ghelardini, C.; Calvani, M.; Nicolai, R.; Mosconi, L.; Toscano, A.; Pacini, A.; Bartolini, A. Neuroprotective effects of acetyl-L-carnitine on neuropathic pain and apoptosis: A role for the nicotinic receptor. J. Neurosci. Res. 2009, 87, 200–207. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.Y.; Park, C.S.; Pyo, M.K.; Yune, T.Y.; Kim, G.W.; Chung, S.H. GS-KG9 ameliorates diabetic neuropathic pain induced by streptozotocin in rats. J. Ginseng Res. 2019, 43, 58–67. [Google Scholar] [CrossRef]
- Nagayach, A.; Patro, N.; Patro, I. Experimentally induced diabetes causes glial activation, glutamate toxicity and cellular damage leading to changes in motor function. Front. Cell Neurosci. 2014, 8, 355. [Google Scholar] [CrossRef] [Green Version]
- Rasoulian, B.; Hajializadeh, Z.; Esmaeili-Mahani, S.; Rashidipour, M.; Fatemi, I.; Kaeidi, A. Neuroprotective and antinociceptive effects of rosemary (Rosmarinus officinalis L.) extract in rats with painful diabetic neuropathy. J. Physiol. Sci. 2019, 69, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obata, H. Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int. J. Mol. Sci. 2017, 18, 2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courteix, C.; Bardin, M.; Chantelauze, C.; Lavarenne, J.; Eschalier, A. Study of the sensitivity of the diabetes-induced pain model in rats to a range of analgesics. Pain 1994, 57, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Max, M.B.; Lynch, S.A.; Muir, J.; Shoaf, S.E.; Smoller, B.; Dubner, R. Effects of desipramine, amitriptyline and fluoxetine on pain in diabetic neuropathy. N. Engl. J. Med. 1992, 326, 1250–1256. [Google Scholar] [CrossRef]
- Kato, D.; Suto, T.; Obata, H.; Saito, S. The efficacy of duloxetine depends on spinal cholinergic plasticity in neuropathic pain model rats. IBRO Neurosci. Rep. 2022, 12, 188–196. [Google Scholar] [CrossRef]
- Bravo, L.; Llorca-Torralba, M.; Berrocoso, E.; Micó, J.A. Monoamines as Drug Targets in Chronic Pain: Focusing on Neuropathic Pain. Front. Neurosci. 2019, 13, 1268. [Google Scholar] [CrossRef]
- Mørk, A.; Montezinho, L.P.; Miller, S.; Trippodi-Murphy, C.; Plath, N.; Li, Y.; Gulinello, M.; Sanchez, C. Vortioxetine (Lu AA21004), a novel multimodal antidepressant, enhances memory in rats. Pharmacol. Biochem. Behav. 2013, 105, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Mørk, A.; Pehrson, A.; Brennum, L.T.; Nielsen, S.M.; Zhong, H.; Lassen, A.B.; Miller, S.; Westrich, L.; Boyle, N.J.; Sánchez, C.; et al. Pharmacological effects of Lu AA21004: A novel multimodal compound for the treatment of major depressive disorder. J. Pharm. Exp. Ther. 2012, 340, 666–675. [Google Scholar] [CrossRef] [Green Version]
- Corrodi, H.; Hanson, L.C. Central effects of an inhibitor of tyrosine hydroxylation. Psychopharmacologia 1966, 10, 116–125. [Google Scholar] [CrossRef]
- Widerlöv, E.; Lewander, T. Inhibition of the in vivo biosynthesis and changes of catecholamine levels in rat brain after alpha-methyl-p-tyrosine; time-and dose-response relationships. Naunyn. Schm. Arch. Pharmacol. 1978, 304, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.X.; Xu, F.Y.; Xu, W.J.; Zhao, Y.; Qu, C.L.; Tang, J.S.; Barry, D.M.; Du, J.Q.; Huo, F.Q. The role of α2 adrenoceptor in mediating noradrenaline action in the ventrolateralorbital cortex on allodynia following spared nerve injury. Exp. Neurol. 2013, 248, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hartung, J.E.; Bortsov, A.V.; Kim, S.; O’Buckley, S.C.; Kozlowski, J.; Nackley, A.G. Sustained stimulation of β2- and β3-adrenergic receptors leads to persistent functional pain and neuroinflammation. Brain Behav. Immun. 2018, 73, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hoshino, H.; Saito, S.; Yang, Y.; Obata, H. Spinal dopaminergic involvement in the antihyperalgesic effect of antidepressants in a rat model of neuropathic pain. Neurosci. Lett. 2017, 649, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Saito, S.; Obata, H. Dexmedetomidine decreases hyperalgesia in neuropathic pain by increasing acetylcholine in the spinal cord. Neurosci. Lett. 2012, 529, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Go, D.; Kim, W.; Lee, G.; Bae, H.; Quan, F.S.; Kim, S.K. Involvement of spinal muscarinic and serotonergic receptors in the anti-allodynic effect of electroacupuncture in rats with oxaliplatin induced neuropathic pain. Korean J. Physiol. Pharmacol. 2016, 20, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Pecikoza, U.; Micov, A.; Tomić, M.; Stepanović-Petrović, R. Eslicarbazepine acetate reduces trigeminal nociception: Possible role of adrenergic, cholinergic and opioid receptors. Life Sci. 2018, 214, 167–175. [Google Scholar] [CrossRef]
- Hunt, S.P.; Pini, A.; Evan, G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 1987, 328, 632–634. [Google Scholar] [CrossRef]
- Harris, J.A. Using c-fos as a neural marker of pain. Brain Res. Bull. 1998, 45, 1–8. [Google Scholar] [CrossRef]
- Coggeshall, R.E. Fos, nociception and the dorsal horn. Prog. Neurobiol. 2005, 77, 299–352. [Google Scholar] [CrossRef]
- Gao, Y.J.; Ji, R.R. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009, 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, H.; Sakamoto, A.; Takeda, S.; Onodera, H.; Imaki, J.; Ogawa, R. A prostaglandin E2 receptor subtype EP1 receptor antagonist (ONO-8711) reduces hyperalgesia, allodynia, and c-fos gene expression in rats with chronic nerve constriction. Anesth. Analg. 2001, 93, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Hossaini, M.; Duraku, L.S.; Kohli, S.K.; Jongen, J.L.; Holstege, J.C. Spinal distribution of c-Fos activated neurons expressing enkephalin in acute and chronic pain models. Brain Res. 2014, 1543, 83–92. [Google Scholar] [CrossRef]
- Siddall, P.J.; Xu, C.L.; Floyd, N.; Keay, K.A. C-fos expression in the spinal cord of rats exhibiting allodynia following contusive spinal cord injury. Brain Res. 1999, 851, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Morgado, C.; Tavares, I. C-fos expression at the spinal dorsal horn of streptozotocin-induced diabetic rats. Diabetes Metab. Res. Rev. 2007, 23, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Ved, N.; Da Vitoria Lobo, M.E.; Bestall, S.M.; Vidueira, C.L.; Beazley-Long, N.; Ballmer-Hofer, K.; Hirashima, M.; Bates, D.O.; Donaldson, L.F.; Hulse, R.P. Diabetes-induced microvascular complications at the level of the spinal cord: A contributing factor in diabetic neuropathic pain. J. Physiol. 2018, 596, 3675–3693. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.R.; Pan, H.L. Hypersensitivity of spinothalamic tract neurons associated with diabetic neuropathic pain in rats. J. Neurophysiol. 2002, 87, 2726–2733. [Google Scholar] [CrossRef] [Green Version]
- Pertovaara, A.; Wei, H.; Kalmari, J.; Ruotsalainen, M. Pain behavior and response properties of spinal dorsal horn neurons following experimental diabetic neuropathy in the rat: Modulation by nitecapone, a COMT inhibitor with antioxidant properties. Exp. Neurol. 2001, 167, 425–434. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turan Yücel, N.; Kandemir, Ü.; Üçel, U.İ.; Demir Özkay, Ü.; Can, Ö.D. Catecholaminergic and Cholinergic Systems Mediate Beneficial Effect of Vortioxetine on Diabetes-Induced Neuropathic Pain. Biomedicines 2023, 11, 1137. https://doi.org/10.3390/biomedicines11041137
Turan Yücel N, Kandemir Ü, Üçel Uİ, Demir Özkay Ü, Can ÖD. Catecholaminergic and Cholinergic Systems Mediate Beneficial Effect of Vortioxetine on Diabetes-Induced Neuropathic Pain. Biomedicines. 2023; 11(4):1137. https://doi.org/10.3390/biomedicines11041137
Chicago/Turabian StyleTuran Yücel, Nazlı, Ümmühan Kandemir, Umut İrfan Üçel, Ümide Demir Özkay, and Özgür Devrim Can. 2023. "Catecholaminergic and Cholinergic Systems Mediate Beneficial Effect of Vortioxetine on Diabetes-Induced Neuropathic Pain" Biomedicines 11, no. 4: 1137. https://doi.org/10.3390/biomedicines11041137