Evaluation of the Antioxidant Activity of Levetiracetam in a Temporal Lobe Epilepsy Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Li2+-Pilocarpine-Induced Temporal Lobe Epilepsy (TLE)
2.3. Monitoring of Spontaneous Behavioral Seizures
2.4. Levetiracetam Treatment
2.5. Measurement of Levetiracetam Blood Levels
2.6. Preparation of Biological Samples
2.7. Antioxidant Markers
2.7.1. SOD Activity Assay
2.7.2. CAT Activity Assay
2.7.3. GPx Activity Assay
2.7.4. GR Activity Assay
2.8. Oxidant Markers
2.8.1. H2O2 Level Determination
2.8.2. Protein Carbonylation Determination
2.8.3. MDA Level Determination
2.8.4. Total Protein Measurement
2.9. In Vitro Scavenging Activity of LEV
2.9.1. DPPH Assay
2.9.2. FRAP Measurement
2.9.3. ORAC Assay
2.9.4. HO• Scavenging Assay
2.9.5. O2•− Scavenging Capacity
2.10. Statistical Analysis
3. Results
3.1. Levetiracetam Blood Levels
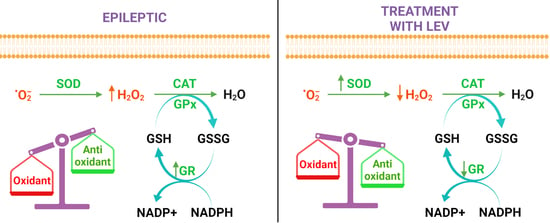
3.2. Antioxidant Markers
3.3. Oxidant Markers
3.4. In Vitro Scavenging Activity of LEV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 16 January 2022).
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falco-Walter, J. Epilepsy-Definition, Classification, Pathophysiology, and Epidemiology. Semin. Neurol. 2020, 40, 617–623. [Google Scholar] [CrossRef]
- Singh, G.; Sander, J.W. The global burden of epilepsy report: Implications for low- and middle-income countries. Epilepsy Behav. 2020, 105, 106949. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Dalton, T.; Pazdernik, T.L.; Wagner, J.; Samson, F.; Andrews, G.K. Temporalspatial patterns of expression of metallothionein-I and -III and other stress related genes in rat brain after kainic acid-induced seizures. Neurochem. Int. 1995, 27, 59–71. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Borowicz-Reutt, K.K.; Czuczwar, S.J. Role of oxidative stress in epileptogenesis and potential implications for therapy. Pharmacol. Rep. 2020, 72, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Vatandousta, S.M.; Ghiasib, R.; Nejadc, G.G.; Shahabi, P. Oxidative Stress and Antioxidant Defense Status in CSF and Blood Content of the WAG/Rij Rat Models Suffering from Absence Epilepsy. J. Exp. Clin. Neurosci. 2016, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Frazzini, V.; Cousyn, L.; Navarro, V. Semiology, EEG, and neuroimaging findings in temporal lobe epilepsies. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 187, pp. 489–518. [Google Scholar] [CrossRef]
- Ramazi, S.; Fahanik-Babaei, J.; Mohamadi-Zarch, S.M.; Tashakori-Miyanroudi, M.; Nourabadi, D.; Nazari-Serenjeh, M.; Roghani, M.; Baluchnejadmojarad, T. Neuroprotective and anticonvulsant effects of sinomenine in kainate rat model of temporal lobe epilepsy: Involvement of oxidative stress, inflammation and pyroptosis. J. Chem. Neuroanat. 2020, 108, 101800. [Google Scholar] [CrossRef]
- Ramazi, S.; Fahanik-Babaei, J.; Mohamadi-Zarch, S.M.; Baluchnejadmojarad, T.; Roghani, M. Paeonol exerts neuroprotective and anticonvulsant effects in intrahippocampal kainate model of temporal lobe epilepsy. J. Chem. Neuroanat. 2022, 124, 102121. [Google Scholar] [CrossRef]
- Tashakori-Miyanroudi, M.; Ramazi, S.; Hashemi, P.; Nazari-Serenjeh, M.; Baluchnejadmojarad, T.; Roghani, M. Acetyl-L-Carnitine Exerts Neuroprotective and Anticonvulsant Effect in Kainate Murine Model of Temporal Lobe Epilepsy. J. Mol. Neurosci. 2022, 72, 1224–1233. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J.; Wang, N.; Mishra, A.; Li, H.; Liu, H.; Fan, Y.; Liu, N.; Wu, Z. Wogonin preventive impact on hippocampal neurodegeneration, inflammation and cognitive defects in temporal lobe epilepsy. Saudi. J. Biol. Sci. 2020, 27, 2149–2156. [Google Scholar] [CrossRef]
- Manford, M. Recent advances in epilepsy. J. Neurol. 2017, 264, 1811–1824. [Google Scholar] [CrossRef] [Green Version]
- Pitkänen, A.; Lukasiuk, K.; Dudek, F.E.; Staley, K.J. Epileptogenesis. Cold Spring Harb. Perspect. Med. 2015, 5, a022822. [Google Scholar] [CrossRef] [Green Version]
- Ashrafi, M.R.; Azizi Malamiri, R.; Shams, S.; Rashidi Ranjbar, N.; Ebrahimi Nasrabadi, S.; Haghi Ashtiani, M.; Saladjegheh, N.; Vakili Zarch, V. Serum Total Antioxidant Capacity of Epileptic Children before and after Monotherapy with Sodium Valproate, Carbamazepine, and Phenobarbital. Iran. J. Child. Neurol. 2018, 12, 24–31. [Google Scholar] [PubMed]
- Morimoto, M.; Hashimoto, T.; Kitaoka, T.; Kyotani, S. Impact of Oxidative Stress and Newer Antiepileptic Drugs on the Albumin and Cortisol Value in Severe Motor and Intellectual Disabilities With Epilepsy. J. Clin. Med. Res. 2018, 10, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finsterer, J.; Scorza, F.A. Effects of antiepileptic drugs on mitochondrial functions, morphology, kinetics, biogenesis, and survival. Epilepsy Res. 2017, 136, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, F.; Malhi, S.M.; Simjee, S.U. Comparative studies on the effects of clinically used anticonvulsants on the oxidative stress biomarkers in pentylenetetrazole-induced kindling model of epileptogenesis in mice. J. Basic. Clin. Physiol. Pharmacol. 2017, 28, 31–42. [Google Scholar] [CrossRef]
- Beltrán-Sarmiento, E.; Arregoitia-Sarabia, C.K.; Floriano-Sánchez, E.; Sandoval-Pacheco, R.; Galván-Hernández, D.E.; Coballase-Urrutia, E.; Carmona-Aparicio, L.; Ramos-Reyna, E.; Rodríguez-Silverio, J.; Cárdenas-Rodríguez, N. Effects of Valproate Monotherapy on the Oxidant-Antioxidant Status in Mexican Epileptic Children: A Longitudinal Study. Oxid. Med. Cell. Longev. 2018, 4, 7954371. [Google Scholar] [CrossRef] [Green Version]
- Steinhoff, B.J.; Staack, A.M. Levetiracetam and brivaracetam: A review of evidence from clinical trials and clinical experience. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419873518. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency (EMA). Keppra. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/keppra#authorisation-details-section (accessed on 12 September 2022).
- Lynch, B.A.; Lambeng, N.; Nocka, K.; Kensel-Hammes, P.; Bajjalieh, S.M.; Matagne, A.; Fuks, B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc. Natl. Acad. Sci. USA 2004, 101, 9861–9866. [Google Scholar] [CrossRef] [Green Version]
- Crepeau, A.Z.; Treiman, D.M. Levetiracetam: A comprehensive review. Expert. Rev. Neurother. 2010, 10, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Contreras-García, I.J.; Cárdenas-Rodríguez, N.; Romo-Mancillas, A.; Bandala, C.; Zamudio, S.R.; Gómez-Manzo, S.; Hernández-Ochoa, B.; Mendoza-Torreblanca, J.G.; Pichardo-Macías, L.A. Levetiracetam Mechanisms of Action: From Molecules to Systems. Pharmaceuticals 2022, 15, 475. [Google Scholar] [CrossRef]
- de Souza, A.G.; Chaves Filho, A.J.M.; Souza Oliveira, J.V.; de Souza, D.A.A.; Lopes, I.S.; de Carvalho, M.A.J.; de Lima, K.A.; Florenço Sousa, F.C.; Mendes Vasconcelos, S.M.; Macedo, D.; et al. Prevention of pentylenetetrazole-induced kindling and behavioral comorbidities in mice by levetiracetam combined with the GLP-1 agonist liraglutide: Involvement of brain antioxidant and BDNF upregulating properties. Biomed. Pharmacother. 2019, 109, 429–439. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.A.; Shaikh, I.A.; Khateeb, M.M.; Habeeb, S.M. Omega 3 polyunsaturated fatty acids enhance the protective effect of levetiracetam against seizures, cognitive impairment and hippocampal oxidative DNA damage in young kindled rats. Pharmacol. Biochem. Behav. 2015, 135, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.A.; Almeida, J.P.; Freitas, R.M.; Nascimento, V.S.; Aguiar, L.M.; Júnior, H.V.; Fonseca, F.N.; Viana, G.S.; Sousa, F.C.; Fonteles, M.M. Effects of levetiracetam in lipid peroxidation level, nitrite-nitrate formation and antioxidant enzymatic activity in mice brain after pilocarpine-induced seizures. Cell. Mol. Neurobiol. 2007, 27, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Dircio-Bautista, M.; Colín-González, A.L.; Aguilera, G.; Maya-López, M.; Villeda-Hernández, J.; Galván-Arzate, S.; García, E.; Túnez, I.; Santamaría, A. The Antiepileptic Drug Levetiracetam Protects Against Quinolinic Acid-Induced Toxicity in the Rat Striatum. Neurotox. Res. 2018, 33, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Ersan, S.; Cigdem, B.; Bakir, D.; Dogan, H.O. Determination of levels of oxidative stress and nitrosative stress in patients with epilepsy. Epilepsy Res. 2020, 164, 106352. [Google Scholar] [CrossRef]
- Pichardo Macías, L.A.; Ramírez Mendiola, B.A.; Contreras García, I.J.; Zamudio Hernández, S.R.; Chávez Pacheco, J.L.; Sánchez Huerta, K.B.; Mendoza Torreblanca, J.G. Effect of levetiracetam on extracellular amino acid levels in the dorsal hippocampus of rats with temporal lobe epilepsy. Epilepsy Res. 2018, 140, 111–119. [Google Scholar] [CrossRef]
- Zamudio, S.R.; Pichardo-Macías, L.A.; Díaz-Villegas, V.; Flores-Navarrete, I.L.; Guzmán-Velázquez, S. Subchronic cerebrolysin treatment alleviates cognitive impairments and dendritic arborization alterations of granular neurons in the hippocampal dentate gyrus of rats with temporal lobe epilepsy. Epilepsy Behav. 2019, 97, 96–104. [Google Scholar] [CrossRef]
- Glien, M.; Brandt, C.; Potschka, H.; Löscher, W. Effects of the novel antiepileptic drug levetiracetam on spontaneous recurrent seizures in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia 2002, 43, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Contreras-García, I.J.; Gómez-Lira, G.; Phillips-Farfán, B.V.; Pichardo-Macías, L.A.; García-Cruz, M.E.; Chávez-Pacheco, J.L.; Mendoza-Torreblanca, J.G. Synaptic Vesicle Protein 2A Expression in Glutamatergic Terminals Is Associated with the Response to Levetiracetam Treatment. Brain. Sci. 2021, 11, 531. [Google Scholar] [CrossRef]
- Oláh, E.; Bacsói, G.; Fekete, J.; Sharma, V.K. Determination of ng/mL levetiracetam using ultra-high-performance liquid chromatography-photodiode absorbance. J. Chromatogr. Sci. 2012, 50, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Meth. Enzymol. 1994, 233, 357–363. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Yam-Canul, P.; Chirino, Y.I.; Sánchez-González, D.J.; Martínez-Martínez, C.M.; Cruz, C.; Medina-Campos, O.N. Protective effects of garlic powder against potassium dichromate-induced oxidative stress and nephrotoxicity. Food. Chem. Toxicol. 2008, 46, 619–627. [Google Scholar] [CrossRef]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.C.; Chaudière, J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef]
- Colín-Barenque, L.; Bizarro-Nevares, P.; González Villalva, A.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Jimenez-Martínez, R.; Rodríguez-Rangel, D.S.; Reséndiz, S.; Fortoul, T.I. Neuroprotective effect of carnosine in the olfactory bulb after vanadium inhalation in a mouse model. Int. J. Exp. Pathol. 2018, 99, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin-Phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Koren, E.; Kohen, R.; Ginsburg, I. Polyphenols enhance total oxidant-scavenging capacities of human blood by binding to red blood cells. Exp. Biol. Med. 2010, 235, 689–699. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food. Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Yan, E.B.; Unthank, J.K.; Castillo-Melendez, M.; Miller, S.L.; Langford, S.J.; Walker, D.W. Novel method for in vivo hydroxyl radical measurement by microdialysis in fetal sheep brain in utero. J. Appl. Physiol 2005, 98, 2304–2310. [Google Scholar] [CrossRef] [Green Version]
- Oberley, L.W.; Spitz, D.R. Assay of superoxide dismutase activity in tumor tissue. Meth. Enzymol. 1984, 105, 457–464. [Google Scholar] [CrossRef]
- Kopan, D.T.; Özçelik, A.A.; Kopan, M.A.; Taysi, S. Assessment of oxidative/nitrosative stress and antioxidant capacity in children with epilepsy. Int. J. Neurosci. 2022, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Yang, L.; Zhang, Y. Modulating the gut microbiota ameliorates spontaneous seizures and cognitive deficits in rats with kainic acid-induced status epilepticus by inhibiting inflammation and oxidative stress. Front. Nutr. 2022, 9, 985841. [Google Scholar] [CrossRef]
- Rehman, Z.; Farooq, T.; Javaid, S.; Ashraf, W.; Fawad Rasool, M.; Samad, N.; Tariq, M.; Muhammad Muneeb Anjum, S.; Sivandzade, F.; Alotaibi, F.; et al. Combination of levetiracetam with sodium selenite prevents pentylenetetrazole-induced kindling and behavioral comorbidities in rats. Saudi. Pharm. J. 2022, 30, 494–507. [Google Scholar] [CrossRef]
- Yue, J.; Xu, R.; Yin, C.; Yang, H.; Zhang, C.; Zhao, D. Negative effects of brain regulatory T cells depletion on epilepsy. Prog. Neurobiol. 2022, 217, 02335. [Google Scholar] [CrossRef]
- Kandeda, A.K.; Nodeina, S.; Mabou, S.T. An aqueous extract of Syzygium cumini protects against kainate-induced status epilepticus and amnesia: Evidence for antioxidant and anti-inflammatory intervention. Metab. Brain. Dis. 2022, 37, 2581–2602. [Google Scholar] [CrossRef]
- Almeida, C.; Pongilio, R.P.; Móvio, M.I.; Higa, G.S.V.; Resende, R.R.; Jiang, J.; Kinjo, E.R.; Kihara, A.H. Distinct Cell-specific Roles of NOX2 and MyD88 in Epileptogenesis. Front. Cell. Dev. Biol. 2022, 10, 926776. [Google Scholar] [CrossRef]
- Olubodun-Obadun, T.G.; Ishola, I.O.; Ben-Azu, B.; Afolayan, O.; Nwose, E.; James, A.B.; Ajayi, A.M.; Umukoro, S.; Adeyemi, O.O. Probable mechanisms involved in the antiepileptic activity of Clerodendrum polycephalum Baker (Labiatae) leaf extract in mice exposed to chemical-induced seizures. J. Food. Biochem. 2022, 46, e14342. [Google Scholar] [CrossRef]
- Maes, M.; Barbosa, D.S.; Almulla, A.F.; Kanchanatawan, B. A Novel Pathway Phenotype of Temporal Lobe Epilepsy and Comorbid Psychiatric Disorders: Results of Precision Nomothetic Medicine. Antioxidants 2022, 11, 803. [Google Scholar] [CrossRef]
- Qian, X.; Wang, Z.R.; Zheng, J.J.; Ding, J.Q.; Zhong, J.G.; Zhang, T.Y.; Li, W.; Zhang, M. Baicalein improves cognitive deficits and hippocampus impairments in temporal lobe epilepsy rats. Brain. Res. 2019, 1714, 111–118. [Google Scholar] [CrossRef]
- Drion, C.M.; van Scheppingen, J.; Arena, A.; Geijtenbeek, K.W.; Kooijman, L.; van Vliet, E.A.; Aronica, E.; Gorter, J.A. Effects of rapamycin and curcumin on inflammation and oxidative stress in vitro and in vivo—In search of potential anti-epileptogenic strategies for temporal lobe epilepsy. J. Neuroinflammation 2018, 15, 212. [Google Scholar] [CrossRef] [Green Version]
- Kiasalari, Z.; Khalili, M.; Shafiee, S.; Roghani, M. The effect of Vitamin E on learning and memory deficits in intrahippocampal kainate-induced temporal lobe epilepsy in rats. Indian. J. Pharmacol. 2016, 48, 11–14. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Saadi, A.; Sheeni, Y.; Shekh-Ahmad, T. Specific inhibition of NADPH oxidase 2 modifies chronic epilepsy. Redox Biol. 2022, 58, 102549. [Google Scholar] [CrossRef]
- Xing, J.; Han, D.; Xu, D.; Li, X.; Sun, L. CREB Protects against Temporal Lobe Epilepsy Associated with Cognitive Impairment by Controlling Oxidative Neuronal Damage. Neurodegener. Dis. 2019, 19, 225–237. [Google Scholar] [CrossRef]
- Pardo-Peña, K.; Sánchez-Lira, A.; Salazar-Sánchez, J.C.; Morales-Villagrán, A. A novel online fluorescence method for in-vivo measurement of hydrogen peroxide during oxidative stress produced in a temporal lobe epilepsy model. NeuroReport 2018, 29, 621–630. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Liang, L.P.; Hellier, J.L.; Staley, K.J.; Patel, M. Mitochondrial DNA damage and impaired base excision repair during epileptogenesis. Neurobiol. Dis. 2008, 30, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, R.; Jaiswal, A.K. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene 1998, 17, 3145–3156. [Google Scholar] [CrossRef] [Green Version]
- Sandouka, S.; Saadi, A.; Singh, P.K.; Olowe, R.; Shekh-Ahmad, T. Nrf2 is predominantly expressed in hippocampal neurons in a rat model of temporal lobe epilepsy. Cell Biosci. 2023, 13, 3. [Google Scholar] [CrossRef]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef] [PubMed]
- Ristić, A.J.; Savić, D.; Sokić, D.; Bogdanović Pristov, J.; Nestorov, J.; Baščarević, V.; Raičević, S.; Savić, S.; Spasojević, I. Hippocampal antioxidative system in mesial temporal lobe epilepsy. Epilepsia 2015, 56, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Rowley, S.; Liang, L.P.; Fulton, R.; Shimizu, T.; Day, B.; Patel, M. Mitochondrial respiration deficits driven by reactive oxygen species in experimental temporal lobe epilepsy. Neurobiol. Dis. 2015, 75, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Ryan, K.; Backos, D.S.; Reigan, P.; Patel, M. Post-translational oxidative modification and inactivation of mitochondrial complex I in epileptogenesis. J. Neurosci. 2012, 32, 11250–11258. [Google Scholar] [CrossRef] [Green Version]
- Kunz, W.S.; Kudin, A.P.; Vielhaber, S.; Blümcke, I.; Zuschratter, W.; Schramm, J.; Beck, H.; Elger, C.E. Mitochondrial complex I deficiency in the epileptic focus of patients with temporal lobe epilepsy. Ann. Neurol. 2000, 48, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Lenkov, D.N.; Volnova, A.B.; Pope, A.R.; Tsytsarev, V. Advantages and limitations of brain imaging methods in the research of absence epilepsy in humans and animal models. J. Neurosci. Methods 2013, 212, 195–202. [Google Scholar] [CrossRef] [PubMed]
- van Luijtelaar, G.; Behr, C.; Avoli, M. Is there such a thing as “generalized” epilepsy? Adv. Exp. Med. Biol. 2014, 813, 81–91. [Google Scholar] [CrossRef]
- Imran, I.; Koch, K.; Schöfer, H.; Lau, H.; Klein, J. Effects of Three Anti-Seizure Drugs on Cholinergic and Metabolic Activity in Experimental Status Epilepticus. J. Pharm. Pharm. Sci. 2019, 22, 340–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osuntokun, O.S.; Abdulwahab, U.F.; Akanji, N.O.; Adedokun, K.I.; Adekomi, A.D.; Olayiwola, G. Anticonvulsant and neuroprotective effects of carbamazepine-levetiracetam adjunctive treatment in convulsive status epilepticus rat model: Inhibition of cholinergic transmission. Neurosci. Lett. 2021, 762, 136167. [Google Scholar] [CrossRef]
- Yang, J.L.; Chen, W.Y.; Chen, Y.P.; Kuo, C.Y.; Chen, S.D. Activation of GLP-1 Receptor Enhances Neuronal Base Excision Repair via PI3K-AKT-Induced Expression of Apurinic/Apyrimidinic Endonuclease 1. Theranostics 2016, 6, 2015–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, H.; Yang, Z. H19 silencing decreases kainic acid-induced hippocampus neuron injury via activating the PI3K/AKT pathway via the H19/miR-206 axis. Exp. Brain. Res. 2022, 240, 2109–2120. [Google Scholar] [CrossRef]
- Zhang, H.; Tao, J.; Zhang, S.; Lv, X. LncRNA MEG3 Reduces Hippocampal Neuron Apoptosis via the PI3K/AKT/mTOR Pathway in a Rat Model of Temporal Lobe Epilepsy. Neuropsychiatr. Dis. Treat 2020, 16, 2519–2528. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, X.; Chang, S.; Wang, Y.; Xu, Y.; Ran, S.; Huang, Z.; Li, P.; Li, J.; Zhang, L.; et al. Totarol prevents neuronal injury in vitro and ameliorates brain ischemic stroke: Potential roles of Akt activation and HO-1 induction. Toxicol. Appl. Pharmacol. 2015, 289, 142–154. [Google Scholar] [CrossRef]
- Miyazaki, I.; Murakami, S.; Torigoe, N.; Kitamura, Y.; Asanuma, M. Neuroprotective effects of levetiracetam target xCT in astrocytes in parkinsonian mice. J. Neurochem. 2016, 136, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.T.; Gauss, K.A. Structure and regulation of the neutrophil respiratory burst oxidase: Comparison with nonphagocyte oxidases. J. Leukoc. Biol. 2004, 76, 760–781. [Google Scholar] [CrossRef] [Green Version]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity. J. Exp. Neurosci. 2016, 10, 23–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboul Ezz, H.S.; Noor, A.E.; Mourad, I.M.; Fahmy, H.; Khadrawy, Y.A. Neurochemical effects of sleep deprivation in the hippocampus of the pilocarpine-induced rat model of epilepsy. Iran. J. Basic. Med. Sci. 2021, 24, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.C.; Shen, H.; Zhang, Y.; Zhu, X.; Wang, J.; Xu, P.; Jiang, D.; Yu, X. The antiepileptic drug levetiracetam promotes neuroblast differentiation and expression of superoxide dismutase in the mouse hippocampal dentate gyrus via PI3K/Akt signalling. Neurosci. Lett. 2018, 662, 84–90. [Google Scholar] [CrossRef]
- Inaba, T.; Miyamoto, N.; Hira, K.; Ueno, Y.; Yamashiro, K.; Watanabe, M.; Shimada, Y.; Hattori, N.; Urabe, T. Protective Role of Levetiracetam Against Cognitive Impairment And Brain White Matter Damage in Mouse prolonged Cerebral Hypoperfusion. Neuroscience 2019, 414, 255–264. [Google Scholar] [CrossRef]
- Rowley, S.; Patel, M. Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free. Radic. Biol. Med. 2013, 62, 121–131. [Google Scholar] [CrossRef] [Green Version]
- E-Comerce of Chemical (ECHEMI) Echemi.com. Available online: https://www.echemi.com/products/pd1805103477-levetiracetam.html (accessed on 12 September 2022).
- Chatgilialoglu, C.; Grzelak, M.; Skotnicki, K.; Filipiak, P.; Kazmierczak, F.; Hug, G.L.; Bobrowski, K.; Marciniak, B. Evaluation of Hydroxyl Radical Reactivity by Thioether Group Proximity in Model Peptide Backbone: Methionine versus S-Methyl-Cysteine. Int. J. Mol. Sci. 2022, 23, 6550. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignacio-Mejía, I.; Contreras-García, I.J.; Mendoza-Torreblanca, J.G.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; García-Cruz, M.E.; Romo-Mancillas, A.; Gómez-Manzo, S.; Bandala, C.; Sánchez-Mendoza, M.E.; et al. Evaluation of the Antioxidant Activity of Levetiracetam in a Temporal Lobe Epilepsy Model. Biomedicines 2023, 11, 848. https://doi.org/10.3390/biomedicines11030848
Ignacio-Mejía I, Contreras-García IJ, Mendoza-Torreblanca JG, Medina-Campos ON, Pedraza-Chaverri J, García-Cruz ME, Romo-Mancillas A, Gómez-Manzo S, Bandala C, Sánchez-Mendoza ME, et al. Evaluation of the Antioxidant Activity of Levetiracetam in a Temporal Lobe Epilepsy Model. Biomedicines. 2023; 11(3):848. https://doi.org/10.3390/biomedicines11030848
Chicago/Turabian StyleIgnacio-Mejía, Iván, Itzel Jatziri Contreras-García, Julieta Griselda Mendoza-Torreblanca, Omar Noel Medina-Campos, José Pedraza-Chaverri, Mercedes Edna García-Cruz, Antonio Romo-Mancillas, Saúl Gómez-Manzo, Cindy Bandala, María Elena Sánchez-Mendoza, and et al. 2023. "Evaluation of the Antioxidant Activity of Levetiracetam in a Temporal Lobe Epilepsy Model" Biomedicines 11, no. 3: 848. https://doi.org/10.3390/biomedicines11030848