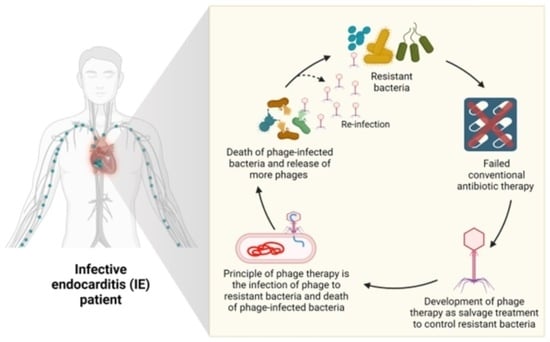
Phage Therapy, a Salvage Treatment for Multidrug-Resistant Bacteria Causing Infective Endocarditis
Abstract
:1. Introduction
2. Bacteriophages: What Are They?
3. History of Phage Therapy
4. Early Recognized Obstacles for Phage Therapy
5. Innovative Studies by Smith and Huggins
6. Phage Therapy: An Updated Strategy
7. Significant Benefits of Phage Therapy over Traditional Antibiotics
8. Clinical Suspicion of IE
9. Microbiological Diagnosis
10. Management and Treatment of IE
10.1. Antibiotic Therapy
10.2. Phage Therapy
10.2.1. Phage Therapy in Combating S. aureus
10.2.2. Phage Therapy in Combating Streptococcus pneumoniae
10.2.3. Phage Therapy in Combating Pseudomonas aeruginosa
10.2.4. Phage Therapy in Combating Enterococci
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Bolger, A.F.; Levison, M.E.; Ferrieri, P.; Gerber, M.A.; Tani, L.Y.; Gewitz, M.H. Infective endocarditis: Diagnosis, antimicrobial therapy, and management of complications: A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: Endorsed by the Infectious Diseases Society of America. Circulation 2005, 111, e394–e434. [Google Scholar]
- Jensen, A.D.; Bundgaard, H.; Butt, J.H.; Bruun, N.E.; Voldstedlund, M.; Torp-Pedersen, C.; Gislason, G.; Iversen, K.; Chamat, S.; Dahl, A. Temporal changes in the incidence of infective endocarditis in Denmark 1997–2017: A nationwide study. Int. J. Cardiol. 2021, 326, 145–152. [Google Scholar] [CrossRef]
- Wang, A.; Gaca, J.G.; Chu, V.H. Management Considerations in Infective Endocarditis: A Review. J. Am. Med. Assoc. 2018, 320, 72–83. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, e521–e643. [Google Scholar] [CrossRef]
- Ambrosioni, J.; Hernandez-Meneses, M.; Téllez, A.; Pericàs, J.; Falces, C.; Tolosana, J.M.; Vidal, B.; Almela, M.; Quintana, E.; Llopis, J.; et al. The Changing Epidemiology of Infective Endocarditis in the Twenty-First Century. Curr. Infect. Dis. Rep. 2017, 19, 21. [Google Scholar] [CrossRef]
- Selton-Suty, C.; Célard, M.; Le Moing, V.; Doco-Lecompte, T.; Chirouze, C.; Iung, B.; Strady, C.; Revest, M.; Vandenesch, F.; Bouvet, A.; et al. Preeminence of Staphylococcus aureus in infective endocarditis: A 1-year population-based survey. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 54, 1230–1239. [Google Scholar] [CrossRef]
- Hajihossainlou, B.; Heidarnia, M.A.; Sharif Kashani, B. Changing pattern of infective endocarditis in Iran: A 16 years survey. Pak. J. Med. Sci. 2013, 29, 85–90. [Google Scholar] [CrossRef]
- Alkhouli, M.; Alqahtani, F.; Alhajji, M.; Berzingi, C.O.; Sohail, M.R. Clinical and Economic Burden of Hospitalizations for Infective Endocarditis in the United States. Mayo Clin. Proc. 2020, 95, 858–866. [Google Scholar] [CrossRef]
- Hubers, S.A.; DeSimone, D.C.; Gersh, B.J.; Anavekar, N.S. Infective Endocarditis: A Contemporary Review. Mayo Clin. Proc. 2020, 95, 982–997. [Google Scholar] [CrossRef]
- Prendergast, B.D. The changing face of infective endocarditis. Heart Br. Card. Soc. 2006, 92, 879–885. [Google Scholar] [CrossRef]
- Rajani, R.; Klein, J.L. Infective endocarditis: A contemporary update. Clin. Med. 2020, 20, 31–35. [Google Scholar] [CrossRef]
- Chen, W.; Sajadi, M.M.; Dilsizian, V. Merits of FDG PET/CT and Functional Molecular Imaging Over Anatomic Imaging with Echocardiography and CT Angiography for the Diagnosis of Cardiac Device Infections. JACC. Cardiovasc. Imaging 2018, 11, 1679–1691. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Bahramian, A.; Shariati, A.; Azimi, T.; Sharahi, J.Y.; Bostanghadiri, N.; Gachkar, L.; Ghalavand, Z.; Chirani, A.S.; Erfanimanesh, S.; Hashemi, A. First report of New Delhi metallo-β-lactamase-6 (NDM-6) among Klebsiella pneumoniae ST147 strains isolated from dialysis patients in Iran. Infect. Genet. Evol. 2019, 69, 142–145. [Google Scholar] [CrossRef]
- Freire-Moran, L.; Aronsson, B.; Manz, C.; Gyssens, I.C.; So, A.D.; Monnet, D.L.; Cars, O.; ECDC-EMA Working Group. Critical shortage of new antibiotics in development against multidrug-resistant bacteria—Time to react is now. Drug Resist. Updates 2011, 14, 118–124. [Google Scholar] [CrossRef]
- Jain, S. Emergence of colistin resistance among gram negative bacteria in urinary tract infections from super specialty hospital of North India. Int. J. Infect. Dis. 2018, 73, 133. [Google Scholar] [CrossRef]
- Mohebi, S.; Hossieni Nave, H.; Norouzi, A.; Kandehkar Gharaman, M.; Taati Moghadam, M. Detection of extended spectrum beta lactamases on class I integron in escherichia coli isolated from clinical samples. J. Maz. Univ. Med. Sci. 2016, 26, 66–76. [Google Scholar]
- Algammal, A.; Hetta, H.F.; Mabrok, M.; Behzadi, P. Emerging multidrug-resistant bacterial pathogens “superbugs”: A rising public health threat. Front. Microbiol. 2023, 14, 1135614. [Google Scholar] [CrossRef]
- Nageeb, W.M.; Hetta, H.F. Pangenome analysis of Corynebacterium striatum: Insights into a neglected multidrug-resistant pathogen. BMC Microbiol. 2023, 23, 252. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Kadmy, I.M.; Ellah, N.H.A.; Shbibe, L.; Battah, B. Nanotechnology-Based Strategies to Combat Multidrug-Resistant Candida auris Infections. Pathogens 2023, 12, 1033. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a promising approach to combat multidrug resistant bacteria: A comprehensive review and future perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Al-Kadmy, I.M.; Ibrahim, S.A.; Al-Saryi, N.; Aziz, S.N.; Besinis, A.; Hetta, H.F. Prevalence of genes involved in colistin resistance in Acinetobacter baumannii: First report from Iraq. Microb. Drug Resist. 2020, 26, 616–622. [Google Scholar] [CrossRef]
- Algammal, A.M.; Abo Hashem, M.E.; Alfifi, K.J.; Al-Otaibi, A.S.; Alatawy, M.; ElTarabili, R.M.; Abd El-Ghany, W.A.; Hetta, H.F.; Hamouda, A.M.; Elewa, A.A. Sequence Analysis, Antibiogram Profile, Virulence and Antibiotic Resistance Genes of XDR and MDR Gallibacterium anatis Isolated from Layer Chickens in Egypt. Infect. Drug Resist. 2022, 15, 4321–4334. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; Alfifi, K.J.; Mabrok, M.; Alatawy, M.; Abdel-Moneam, D.A.; Alghamdi, S.; Azab, M.M.; Ibrahim, R.A.; Hetta, H.F.; El-Tarabili, R.M. Newly Emerging MDR B. cereus in Mugil seheli as the First Report Commonly Harbor nhe, hbl, cytK, and pc-plc Virulence Genes and bla1, bla2, tetA, and ermA Resistance Genes. Infect Drug Resist. 2022, 15, 2167–2185. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; Hashem, H.R.; Al-Otaibi, A.S.; Alfifi, K.J.; El-Dawody, E.M.; Mahrous, E.; Hetta, H.F.; El-Kholy, A.W.; Ramadan, H.; El-Tarabili, R.M. Emerging MDR-Mycobacterium avium subsp. avium in house-reared domestic birds as the first report in Egypt. BMC Microbiol. 2021, 21, 237. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; Hashem, H.R.; Alfifi, K.J.; Hetta, H.F.; Sheraba, N.S.; Ramadan, H.; El-Tarabili, R.M. atpD gene sequencing, multidrug resistance traits, virulence-determinants, and antimicrobial resistance genes of emerging XDR and MDR-Proteus mirabilis. Sci. Rep. 2021, 11, 9476. [Google Scholar] [CrossRef]
- Algammal, A.M.; Hetta, H.F.; Batiha, G.E.; Hozzein, W.N.; El Kazzaz, W.M.; Hashem, H.R.; Tawfik, A.M.; El-Tarabili, R.M. Virulence-determinants and antibiotic-resistance genes of MDR-E. coli isolated from secondary infections following FMD-outbreak in cattle. Sci. Rep. 2020, 10, 19779. [Google Scholar]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.-S.; El Nahhas, N.; Mabrok, M.A. Methicillin-Resistant Staphylococcus aureus (MRSA): One health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infect. Drug Resist. 2020, 13, 3255–3265. [Google Scholar] [CrossRef]
- Algammal, A.M.; Mabrok, M.; Sivaramasamy, E.; Youssef, F.M.; Atwa, M.H.; El-Kholy, A.W.; Hetta, H.F.; Hozzein, W.N. Emerging MDR-Pseudomonas aeruginosa in fish commonly harbor oprL and toxA virulence genes and blaTEM, blaCTX-M, and tetA antibiotic-resistance genes. Sci. Rep. 2020, 10, 15961. [Google Scholar] [CrossRef]
- El-Kazzaz, W.; Metwally, L.; Yahia, R.; Al-Harbi, N.; El-Taher, A.; Hetta, H.F. Antibiogram, prevalence of OXA carbapenemase encoding genes, and RAPD-genotyping of multidrug-resistant Acinetobacter baumannii incriminated in hidden community-acquired infections. Antibiotics 2020, 9, 603. [Google Scholar] [CrossRef]
- El-Masry, E.A.; Taher, I.; Hetta, H.F.; Eldahdouh, S.S. Pulmonary tuberculosis susceptibility and association with Toll-Like receptor 2 Arg753Gln polymorphism. J. Infect. Dev. Ctries. 2022, 16, 125–133. [Google Scholar] [CrossRef]
- El-Mokhtar, M.A.; Hetta, H.F. Ambulance vehicles as a source of multidrug-resistant infections: A multicenter study in Assiut City, Egypt. Infect. Drug Resist. 2018, 11, 587–594. [Google Scholar] [CrossRef]
- Elkhawaga, A.A.; Hetta, H.F.; Osman, N.S.; Hosni, A.; El-Mokhtar, M.A. Emergence of Cronobacter sakazakii in cases of neonatal sepsis in upper Egypt: First report in North Africa. Front. Microbiol. 2020, 11, 215. [Google Scholar] [CrossRef]
- Kareem, S.M.; Al-Kadmy, I.M.; Kazaal, S.S.; Ali, A.N.M.; Aziz, S.N.; Makharita, R.R.; Algammal, A.M.; Al-Rejaie, S.; Behl, T.; Batiha, G.E.-S. Detection of gyra and parc mutations and prevalence of plasmid-mediated quinolone resistance genes in Klebsiella pneumoniae. Infect. Drug Resist. 2021, 14, 555–563. [Google Scholar] [CrossRef]
- Makharita, R.R.; El-Kholy, I.; Hetta, H.F.; Abdelaziz, M.H.; Hagagy, F.I.; Ahmed, A.A.; Algammal, A.M. Antibiogram and genetic characterization of carbapenem-resistant gram-negative pathogens incriminated in healthcare-associated infections. Infect. Drug Resist. 2020, 13, 3991–4002. [Google Scholar] [CrossRef]
- Nageeb, W.M.; Hetta, H.F. The predictive potential of different molecular markers linked to amikacin susceptibility phenotypes in Pseudomonas aeruginosa. PLoS ONE 2022, 17, e0267396. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, M.; Norouzi, A.; Taghadosi, R.; Mohebi, S.; Mohammadi, M.; Hasanzade, A.; Moghadam, M.T. Prevalence of qnr, intI, and intII genes in extendedspectrum beta-lactamase (ESBL)-producing Escherichia coli isolated from clinical samples in Iran. Trop. J. Pharm. Res. 2017, 16, 141–147. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Wei, W.; Hu, X.-X.; Tang, S.; Pang, J.; You, X.-F.; Fan, T.-Y.; Wang, Y.-X.; Song, D.-Q. Evolution and antibacterial evaluation of 8-hydroxy-cycloberberine derivatives as a novel family of antibacterial agents against MRSA. Molecules 2019, 24, 984. [Google Scholar] [CrossRef]
- Nageeb, W.M.; AlHarbi, N.; Alrehaili, A.A.; Zakai, S.A.; Elfadadny, A.; Hetta, H.F. Global genomic epidemiology of chromosomally mediated non-enzymatic carbapenem resistance in Acinetobacter baumannii: On the way to predict and modify resistance. Front. Microbiol. 2023, 14, 1271733. [Google Scholar] [CrossRef]
- Abd El-Aziz, F.E.-Z.A.; Hetta, H.F.; Abdelhamid, B.N.; Abd Ellah, N.H. Antibacterial and wound-healing potential of PLGA/spidroin nanoparticles: A study on earthworms as a human skin model. Nanomedicine 2022, 17, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Baky, R.M.; Farhan, S.M.; Ibrahim, R.A.; Mahran, K.M.; Hetta, H.F. Antimicrobial resistance pattern and molecular epidemiology of ESBL and MBL producing Acinetobacter baumannii isolated from hospitals in Minia, Egypt. Alex. J. Med. 2020, 56, 4–13. [Google Scholar] [CrossRef]
- Abd El-Baky, R.M.; Masoud, S.M.; Mohamed, D.S.; Waly, N.G.; Shafik, E.A.; Mohareb, D.A.; Elkady, A.; Elbadr, M.M.; Hetta, H.F. Prevalence and some possible mechanisms of colistin resistance among multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosaInfect. Drug Resist. 2020, 13, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Baky, R.M.; Sandle, T.; John, J.; Abuo-Rahma, G.E.-D.A.; Hetta, H.F. A novel mechanism of action of ketoconazole: Inhibition of the NorA efflux pump system and biofilm formation in multidrug-resistant Staphylococcus aureus. Infect. Drug Resist. 2019, 12, 1703–1718. [Google Scholar] [CrossRef] [PubMed]
- Boluki, E.; Kazemian, H.; Peeridogaheh, H.; Alikhani, M.Y.; Shahabi, S.; Beytollahi, L.; Ghorbanzadeh, R. Antimicrobial activity of photodynamic therapy in combination with colistin against a pan-drug resistant Acinetobacter baumannii isolated from burn patient. Photodiagnosis Photodyn. Ther. 2017, 18, 1–5. [Google Scholar] [CrossRef]
- Alster, C.J.; Baas, P.; Wallenstein, M.D.; Johnson, N.G.; Von Fischer, J.C. Temperature sensitivity as a microbial trait using parameters from macromolecular rate theory. Front. Microbiol. 2016, 7, 1821. [Google Scholar] [CrossRef] [PubMed]
- Sharahi, J.Y.; Azimi, T.; Shariati, A.; Safari, H.; Tehrani, M.K.; Hashemi, A. Advanced strategies for combating bacterial biofilms. J. Cell. Physiol. 2019, 234, 14689–14708. [Google Scholar] [CrossRef]
- Burrowes, B.; Harper, D.R.; Anderson, J.; McConville, M.; Enright, M.C. Bacteriophage therapy: Potential uses in the control of antibiotic-resistant pathogens. Expert Rev. Anti-Infect. Ther. 2011, 9, 775–785. [Google Scholar] [CrossRef]
- Kamal, F.; Dennis, J.J. Burkholderia cepacia complex phage-antibiotic synergy (PAS): Antibiotics stimulate lytic phage activity. Appl. Environ. Microbiol. 2015, 81, 1132–1138. [Google Scholar] [CrossRef]
- Knezevic, P.; Curcin, S.; Aleksic, V.; Petrusic, M.; Vlaski, L. Phage-antibiotic synergism: A possible approach to combatting Pseudomonas aeruginosaRes. Microbiol. 2013, 164, 55–60. [Google Scholar] [CrossRef]
- Mapes, A.C.; Trautner, B.W.; Liao, K.S.; Ramig, R.F. Development of expanded host range phage active on biofilms of multi-drug resistantPseudomonas aeruginosa. Bacteriophage 2016, 6, e1096995. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Huang, G.; Zhang, Y.; Jiang, B.; Yang, Z.; Dong, Z.; You, B.; Yuan, Z.; Hu, F.; Zhao, Y. Phage Abp1 rescues human cells and mice from infection by pan-drug resistant Acinetobacter baumannii. Cell. Physiol. Biochem. 2017, 44, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.D.; Lyrawati, D. Interactions between Bacteriophages and Eukaryotic Cells. Scientifica 2020, 2020, 3589316. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M. Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers. MBio 2017, 8, e01874-17. [Google Scholar] [CrossRef]
- Bolocan, A.S.; Callanan, J.; Forde, A.; Ross, P.; Hill, C. Phage therapy targeting Escherichia coli—A story with no end? FEMS Microbiol. Lett. 2016, 363, fnw256. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage therapy: A renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef]
- Brussow, H. Phage therapy: The Escherichia coli experience. Microbiology 2005, 151, 2133–2140. [Google Scholar] [CrossRef]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in Aquatic Ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef]
- Mushegian, A. Are there 1031 virus particles on earth, or more, or fewer? J. Bacteriol. 2020, 202, e00052-20. [Google Scholar] [CrossRef]
- Hendrix, R.W.; Hatfull, G.F.; Ford, M.E.; Smith, M.C.; Burns, R.N. Evolutionary relationships among diverse bacteriophages and prophages: All the world’s a phage. In Horizontal Gene Transfer; Elsevier: Amsterdam, The Netherlands, 2002; pp. 133–140, V–VI. [Google Scholar]
- Hatfull, G.F. Actinobacteriophages: Genomics, dynamics, and applications. Annu. Rev. Virol. 2020, 7, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Knowles, B.; Silveira, C.; Bailey, B.; Barott, K.; Cantu, V.; Cobián-Güemes, A.; Coutinho, F.; Dinsdale, E.; Felts, B.; Furby, K. Lytic to temperate switching of viral communities. Nature 2016, 531, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef]
- D’Herelle, M. Sur un microbe invisible antagoniste des bacilles dysentériques. Acta Kravsi 1961, 165, 373–375. [Google Scholar]
- Ho, K. Bacteriophage therapy for bacterial infections. Rekindling a memory from the pre-antibiotics era. Perspect. Biol. Med. 2001, 44, 1–16. [Google Scholar] [CrossRef]
- D’Herelle, F. The Bacteriophage and Its Behavior; Williams & Wilkins: Philadelphia, PA, USA, 1926. [Google Scholar]
- Summers, W.C. Cholera and plague in India: The bacteriophage inquiry of 1927–1936. J. Hist. Med. Allied Sci. 1993, 48, 275–301. [Google Scholar] [CrossRef]
- D’Herelle, F.; Malone, R.; Lahiri, M. Studies on Asiatic cholera. In Indian Medical Research Memoirs; Thacker, Spink Co.: Calcutta, India, 1930. [Google Scholar]
- Cowie, D.; Hicks, W. Observations on the bacteriophage III. J. Lab. Clin. Med. 1932, 17, 685. [Google Scholar]
- Krestownikowa, W.; Gubin, W. Die Verteilung and die Ausscheidung von Bak-teriophagen im Meerschweinchen-organismus bei subkutaner Applicationsart. J. Microbiol. Patolog. I. Infekzionnich Boles. 1925, 1. [Google Scholar]
- Luria, S.E.; Delbrück, M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 1943, 28, 491–511. [Google Scholar] [CrossRef]
- Riding, D. Acute bacillary dysentery in Khartoum province, Sudan, with special reference to bacteriophage treatment: Bacteriological investigation. Epidemiol. Infect. 1930, 30, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Krueger, A.P.; Scribner, E.J. The bacteriophage: Its nature and its therapeutic use. J. Am. Med. Assoc. 1941, 116, 2269–2277. [Google Scholar] [CrossRef]
- Smith, H.W.; Huggins, M. Successful treatment of experimental Escherichia coli infections in mice using phage: Its general superiority over antibiotics. Microbiology 1982, 128, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.W.; Huggins, M.B.; Shaw, K.M. Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. Microbiology 1987, 133, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Merril, C.R.; Biswas, B.; Carlton, R.; Jensen, N.C.; Creed, G.J.; Zullo, S.; Adhya, S. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. USA 1996, 93, 3188–3192. [Google Scholar] [CrossRef] [PubMed]
- Soothill, J. Treatment of experimental infections of mice with bacteriophages. J. Med. Microbiol. 1992, 37, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.; Levin, B.R.; DeRouin, T.; Walker, N.; Bloch, C.A. Dynamics of success and failure in phage and antibiotic therapy in experimental infections. BMC Microbiol. 2002, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Immune evasion by staphylococci. Nat. Rev. Microbiol. 2005, 3, 948–958. [Google Scholar] [CrossRef]
- Geisinger, E.; Isberg, R.R. Antibiotic Modulation of Capsular Exopolysaccharide and Virulence in Acinetobacter baumannii. PLoS Pathog. 2015, 11, e1004691. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Bishop-Lilly, K.A.; Plaut, R.D.; Chen, P.E.; Akmal, A.; Willner, K.M.; Butani, A.; Dorsey, S.; Mokashi, V.; Mateczun, A.J.; Chapman, C. Whole genome sequencing of phage resistant Bacillus anthracismutants reveals an essential role for cell surface anchoring protein CsaB in phage AP50c adsorption. Virol. J. 2012, 9, 246. [Google Scholar] [CrossRef]
- Choi, Y.; Shin, H.; Lee, J.-H.; Ryu, S. Identification and Characterization of a Novel Flagellum-Dependent Salmonella-Infecting Bacteriophage, iEPS5. Appl. Environ. Microbiol. 2013, 79, 4829–4837. [Google Scholar] [CrossRef] [PubMed]
- Davison, S.; Couture-Tosi, E.; Candela, T.; Mock, M.; Fouet, A. Identification of the Bacillus anthracis γ; Phage Receptor. J. Bacteriol. 2005, 187, 6742–6749. [Google Scholar] [CrossRef]
- Lood, R.; Winer, B.Y.; Pelzek, A.J.; Diez-Martinez, R.; Thandar, M.; Euler, C.W.; Schuch, R.; Fischetti, V.A. Novel Phage Lysin Capable of Killing the Multidrug-Resistant Gram-Negative Bacterium Acinetobacter baumannii in a Mouse Bacteremia Model. Antimicrob. Agents Chemother. 2015, 59, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Nakonieczna, A.; Cooper, C.J.; Gryko, R. Bacteriophages and bacteriophage-derived endolysins as potential therapeutics to combat Gram-positive spore forming bacteria. J. Appl. Microbiol. 2015, 119, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Moreirinha, C.; Teles, L.; Rocha, R.J.M.; Calado, R.; Romalde, J.L.; Nunes, M.L.; Almeida, A. Application of phage therapy during bivalve depuration improves Escherichia coli decontamination. Food Microbiol. 2017, 61, 102–112. [Google Scholar] [CrossRef]
- Pabary, R.; Singh, C.; Morales, S.; Bush, A.; Alshafi, K.; Bilton, D.; Alton, E.W.F.W.; Smithyman, A.; Davies, J.C. Antipseudomonal Bacteriophage Reduces Infective Burden and Inflammatory Response in Murine Lung. Antimicrob. Agents Chemother. 2016, 60, 744–751. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Weber-Dąbrowska, B.; Fortuna, W.; Letkiewicz, S.; Rogóż, P.; Jończyk-Matysiak, E.; Dąbrowska, K.; Majewska, J.; Borysowski, J. Phage Therapy: Combating Infections with Potential for Evolving from Merely a Treatment for Complications to Targeting Diseases. Front. Microbiol. 2016, 7, 1515. [Google Scholar] [CrossRef]
- De Sordi, L.; Khanna, V.; Debarbieux, L. The Gut Microbiota Facilitates Drifts in the Genetic Diversity and Infectivity of Bacterial Viruses. Cell Host Microbe 2017, 22, 801–808.e803. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef]
- Caliendo, A.M.; Gilbert, D.N.; Ginocchio, C.C.; Hanson, K.E.; May, L.; Quinn, T.C.; Tenover, F.C.; Alland, D.; Blaschke, A.J.; Bonomo, R.A.; et al. Better tests, better care: Improved diagnostics for infectious diseases. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 57 (Suppl. 3), S139–S170. [Google Scholar] [CrossRef]
- Taati Moghadam, M.; Amirmozafari, N.; Shariati, A.; Hallajzadeh, M.; Mirkalantari, S.; Khoshbayan, A.; Masjedian Jazi, F. How Phages Overcome the Challenges of Drug Resistant Bacteria in Clinical Infections. Infect. Drug Resist. 2020, 13, 45–61. [Google Scholar] [CrossRef]
- Wittebole, X.; De Roock, S.; Opal, S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 2014, 5, 226–235. [Google Scholar] [CrossRef]
- Shen, G.-H.; Wang, J.-L.; Wen, F.-S.; Chang, K.-M.; Kuo, C.-F.; Lin, C.-H.; Luo, H.-R.; Hung, C.-H. Isolation and Characterization of φkm18p, a Novel Lytic Phage with Therapeutic Potential against Extensively Drug Resistant Acinetobacter baumannii. PLoS ONE 2012, 7, e46537. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, F.L.; Costa, A.R.; Kluskens, L.D.; Azeredo, J. Revisiting phage therapy: New applications for old resources. Trends Microbiol. 2015, 23, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Mirrett, S.; Reller, L.B.; Weinstein, M.P. Detection of bloodstream infections in adults: How many blood cultures are needed? J. Clin. Microbiol. 2007, 45, 3546–3548. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Yallowitz, A.W.; Decker, L.C. Infectious Endocarditis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hoen, B.; Duval, X. Clinical practice. Infective endocarditis. N. Engl. J. Med. 2013, 368, 1425–1433. [Google Scholar] [CrossRef]
- Kafetz, K.; Atkin, N. Diagnosing dying in the acute hospital setting. Clin. Med. 2009, 9, 398–399; author reply 399. [Google Scholar] [CrossRef]
- Gavaldà, J.; Len, O.; Miró, J.M.; Muñoz, P.; Montejo, M.; Alarcón, A.; de la Torre-Cisneros, J.; Peña, C.; Martínez-Lacasa, X.; Sarria, C.; et al. Brief communication: Treatment of Enterococcus faecalis endocarditis with ampicillin plus ceftriaxone. Ann. Intern. Med. 2007, 146, 574–579. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e1159–e1195. [Google Scholar] [CrossRef]
- Asgeirsson, H.; Thalme, A.; Weiland, O. Staphylococcus aureus bacteraemia and endocarditis–epidemiology and outcome: A review. Infect. Dis. 2018, 50, 175–192. [Google Scholar] [CrossRef]
- Petrovic Fabijan, A.; Lin, R.C.; Ho, J.; Maddocks, S.; Ben Zakour, N.L.; Iredell, J.R.; Westmead Bacteriophage Therapy Team. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Gilbey, T.; Ho, J.; Cooley, L.A.; Fabijan, A.P.; Iredell, J.R. Adjunctive bacteriophage therapy for prosthetic valve endocarditis due to Staphylococcus aureus. Med. J. Aust. 2019, 211, 142–143.e1. [Google Scholar] [CrossRef]
- Save, J.; Que, Y.A.; Entenza, J.M.; Kolenda, C.; Laurent, F.; Resch, G. Bacteriophages Combined with Subtherapeutic Doses of Flucloxacillin Act Synergistically Against Staphylococcus aureus Experimental Infective Endocarditis. J. Am. Heart Assoc. 2022, 11, e023080. [Google Scholar] [CrossRef] [PubMed]
- Save, J.; Que, Y.-A.; Entenza, J.; Resch, G. Subtherapeutic Doses of Vancomycin Synergize with Bacteriophages for Treatment of Experimental Methicillin-Resistant Staphylococcus aureus Infective Endocarditis. Viruses 2022, 14, 1792. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.B.; Gaukel, E.; Kerzee, N.; Borroto-Esoda, K.; Lowry, S.; Xiong, Y.Q.; Abdelhady, W.; Bayer, A.S. Efficacy of Antistaphylococcal Lysin LSVT-1701 in Combination with Daptomycin in Experimental Left-Sided Infective Endocarditis Due to Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2021, 65, e0050821. [Google Scholar] [CrossRef]
- Aslam, S.; Pretorius, V.; Lehman, S.M.; Morales, S.; Schooley, R.T. Novel bacteriophage therapy for treatment of left ventricular assist device infection. J. Heart Lung Transplant. 2019, 38, 475–476. [Google Scholar] [CrossRef]
- Moorthy, G.S.; Greenberg, R.G.; Hornik, C.D.; Cassino, C.; Ghahramani, P.; Kumar, K.R.; Fowler, V.G.; Cohen-Wolkowiez, M. Safety and Pharmacokinetics of Exebacase in an Infant with Disseminated Staphylococcus aureus Infection. Clin. Infect. Dis. 2021, 75, 338–341. [Google Scholar] [CrossRef]
- Entenza, J.M.; Loeffler, J.M.; Grandgirard, D.; Fischetti, V.A.; Moreillon, P. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob. Agents Chemother. 2005, 49, 4789–4792. [Google Scholar] [CrossRef]
- Oechslin, F.; Piccardi, P.; Mancini, S.; Gabard, J.; Moreillon, P.; Entenza, J.M.; Resch, G.; Que, Y.A. Synergistic Interaction Between Phage Therapy and Antibiotics Clears Pseudomonas Aeruginosa Infection in Endocarditis and Reduces Virulence. J. Infect. Dis. 2017, 215, 703–712. [Google Scholar] [CrossRef]
- Bolocan, A.S.; Upadrasta, A.; de Almeida Bettio, P.H.; Clooney, A.G.; Draper, L.A.; Ross, R.P.; Hill, C. Evaluation of Phage Therapy in the Context of Enterococcus faecalis and Its Associated Diseases. Viruses 2019, 11, 366. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, E.; Coyle, E.A.; Kaatz, G.W.; Zervos, M.J.; Rybak, M.J. Comparison of a rabbit model of bacterial endocarditis and an in vitro infection model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 2000, 44, 1921–1924. [Google Scholar] [CrossRef] [PubMed]
- McGrath, B.J.; Kang, S.L.; Kaatz, G.W.; Rybak, M.J. Bactericidal activities of teicoplanin, vancomycin, and gentamicin alone and in combination against Staphylococcus aureus in an in vitro pharmacodynamic model of endocarditis. Antimicrob. Agents Chemother. 1994, 38, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, L.; Gelman, D.; Shlezinger, M.; Dessal, A.L.; Coppenhagen-Glazer, S.; Beyth, N.; Hazan, R. Defeating antibiotic-and phage-resistant Enterococcus faecalis using a phage cocktail in vitro and in a clot model. Front. Microbiol. 2018, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Coyne, A.J.K.; Stamper, K.; Ghali, A.E.; Kebriaei, R.; Biswas, B.; Wilson, M.; Deschenes, M.V.; Tran, T.T.; Arias, C.A.; Rybak, M.J. Phage-Antibiotic Cocktail Rescues Daptomycin and Phage Susceptibility against Daptomycin-Nonsusceptible Enterococcus faecium in a Simulated Endocardial Vegetation Ex Vivo Model. Microbiol. Spectr. 2023, 11, e00340-23. [Google Scholar] [CrossRef]
- Fowler, V., Jr.; Miro, J.; Hoen, B.; Cabell, C.; Abrutyn, E.; Rubinstein, E.; Corey, G.; Spelman, D.; Bradley, S.; Barsic, B. Investigators ICE: Staphylococcus aureus endocarditis: A consequence of medical progress. J. Am. Med. Assoc. 2005, 293, 3012–3021. [Google Scholar] [CrossRef]
- Wang, A.; Athan, E.; Pappas, P.A.; Fowler, V.G.; Olaison, L.; Paré, C.; Almirante, B.; Muñoz, P.; Rizzi, M.; Naber, C. Contemporary clinical profile and outcome of prosthetic valve endocarditis. J. Am. Med. Assoc. 2007, 297, 1354–1361. [Google Scholar] [CrossRef]
- Chirouze, C.; Alla, F.; Fowler, V.G., Jr.; Sexton, D.J.; Corey, G.R.; Chu, V.H.; Wang, A.; Erpelding, M.-L.; Durante-Mangoni, E.; Fernández-Hidalgo, N. Impact of early valve surgery on outcome of Staphylococcus aureus prosthetic valve infective endocarditis: Analysis in the International Collaboration of Endocarditis–Prospective Cohort Study. Clin. Infect. Dis. 2015, 60, 741–749. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, W.; Zhong, Q.; Chen, Q.; He, X.; Baker, J.L.; Xiong, K.; Jin, X.; Wang, J.; Hu, F. Development of a bacteriophage cocktail to constrain the emergence of phage-resistant Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 327. [Google Scholar]
- Rose, T.; Verbeken, G.; De Vos, D.; Merabishvili, M.; Vaneechoutte, M.; Lavigne, R.; Jennes, S.; Zizi, M.; Pirnay, J.-P. Experimental phage therapy of burn wound infection: Difficult first steps. Int. J. Burn. Trauma 2014, 4, 66. [Google Scholar]
- Gupta, P.; Singh, H.S.; Shukla, V.K.; Nath, G.; Bhartiya, S.K. Bacteriophage therapy of chronic nonhealing wound: Clinical study. Int. J. Low. Extrem. Wounds 2019, 18, 171–175. [Google Scholar] [CrossRef]
- Doub, J.B.; Ng, V.Y.; Johnson, A.J.; Slomka, M.; Fackler, J.; Horne, B.A.; Brownstein, M.J.; Henry, M.; Malagon, F.; Biswas, B. Salvage bacteriophage therapy for a chronic MRSA prosthetic joint infection. Antibiotics 2020, 9, 241. [Google Scholar] [CrossRef]
- Ferry, T.; Kolenda, C.; Batailler, C.; Gustave, C.-A.; Lustig, S.; Malatray, M.; Fevre, C.; Josse, J.; Petitjean, C.; Chidiac, C. Phage therapy as adjuvant to conservative surgery and antibiotics to salvage patients with relapsing S. aureus prosthetic knee infection. Front. Med. 2020, 7, 570572. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Ali, E.M.; Kandeel, M.; Venugopala, K.N.; Nair, A.B.; Greish, K.; El-Daly, M. Screening and molecular docking of novel benzothiazole derivatives as potential antimicrobial agents. Antibiotics 2020, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Lampley, E.; Wooten, D.; Karris, M.; Benson, C.; Strathdee, S.; Schooley, R.T. Lessons learned from the first 10 consecutive cases of intravenous bacteriophage therapy to treat multidrug-resistant bacterial infections at a single center in the united states. Open Forum Infect. Dis. 2020, 7, ofaa389. [Google Scholar] [CrossRef] [PubMed]
- Fadlallah, A.; Chelala, E.; Legeais, J.-M. Corneal infection therapy with topical bacteriophage administration. Open Ophthalmol. J. 2015, 9, 167–168. [Google Scholar] [CrossRef]
- Que, Y.A.; Moreillon, P. Infective endocarditis. Nat. Rev. Cardiol. 2011, 8, 322–336. [Google Scholar] [CrossRef]
- De Kraker, M.E.; Wolkewitz, M.; Davey, P.G.; Koller, W.; Berger, J.; Nagler, J.; Icket, C.; Kalenic, S.; Horvatic, J.; Seifert, H.; et al. Clinical impact of antimicrobial resistance in European hospitals: Excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob. Agents Chemother. 2011, 55, 1598–1605. [Google Scholar] [CrossRef]
- Neely, M.N.; Youn, G.; Jones, B.; Jelliffe, R.W.; Drusano, G.L.; Rodvold, K.A.; Lodise, T.P. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob. Agents Chemother. 2014, 58, 309–316. [Google Scholar] [CrossRef]
- Pfeltz, R.; Wilkinson, B. The escalating challenge of vancomycin resistance in Staphylococcus aureus. Curr. Drug Targets-Infect. Disord. 2004, 4, 273–294. [Google Scholar] [CrossRef]
- Valente, L.; Prazak, J.; Que, Y.-A.; Cameron, D.R. Progress and pitfalls of bacteriophage therapy in critical care: A concise definitive review. Crit. Care Explor. 2021, 3, e0351. [Google Scholar] [CrossRef]
- Park, S.C.; Shimamura, I.; Fukunaga, M.; Mori, K.I.; Nakai, T. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl. Environ. Microbiol. 2000, 66, 1416–1422. [Google Scholar] [CrossRef]
- Arias, C.A.; Contreras, G.A.; Murray, B.E. Management of multidrug-resistant enterococcal infections. Clin. Microbiol. Infect. 2010, 16, 555–562. [Google Scholar] [CrossRef]
- Sava, I.G.; Heikens, E.; Huebner, J. Pathogenesis and immunity in enterococcal infections. Clin. Microbiol. Infect. 2010, 16, 533–540. [Google Scholar] [CrossRef]
- Berg, R.D. The indigenous gastrointestinal microflora. Trends Microbiol. 1996, 4, 430–435. [Google Scholar] [CrossRef]
- Facklam, R.R.; Carvalho, M.d.G.S.; Teixeira, L.M. History, taxonomy, biochemical characteristics, and antibiotic susceptibility testing of enterococci. In The Enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance; ASM Press: Washington, DC, USA, 2002; pp. 1–54. [Google Scholar]
- Simonsen, G.; Småbrekke, L.; Monnet, D.; Sørensen, T.; Møller, J.; Kristinsson, K.; Lagerqvist-Widh, A.; Torell, E.; Digranes, A.; Harthug, S. Prevalence of resistance to ampicillin, gentamicin and vancomycin in Enterococcus faecalis and Enterococcus faecium isolates from clinical specimens and use of antimicrobials in five Nordic hospitals. J. Antimicrob. Chemother. 2003, 51, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Kristich, C.J.; Rice, L.B.; Arias, C.A. Enterococcal infection—Treatment and antibiotic resistance. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Beganovic, M.; Luther, M.K.; Rice, L.B.; Arias, C.A.; Rybak, M.J.; LaPlante, K.L. A review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin. Infect. Dis. 2018, 67, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Jung, N.; Cornely, O.A.; Rybniker, J.; Fätkenheuer, G. Combination antimicrobial therapy for Enterococcus faecalis infective endocarditis. Clin. Infect. Dis. 2019, 69, 900. [Google Scholar] [CrossRef]
- Dalmasso, M.; De Haas, E.; Neve, H.; Strain, R.; Cousin, F.J.; Stockdale, S.R.; Ross, R.P.; Hill, C. Isolation of a novel phage with activity against Streptococcus mutans biofilms. PLoS ONE 2015, 10, e0138651. [Google Scholar] [CrossRef]
- Abdulamir, A.S.; Jassim, S.A.; Hafidh, R.R.; Bakar, F.A. The potential of bacteriophage cocktail in eliminating Methicillin-resistant Staphylococcus aureus biofilms in terms of different extracellular matrices expressed by PIA, ciaA-D and FnBPA genes. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Nazareth, N.; Magro, F.; Machado, E.; Ribeiro, T.G.; Martinho, A.; Rodrigues, P.; Alves, R.; Macedo, G.N.; Gracio, D.; Coelho, R. Prevalence of Mycobacterium avium subsp. paratuberculosis and Escherichia coli in blood samples from patients with inflammatory bowel disease. Med. Microbiol. Immunol. 2015, 204, 681–692. [Google Scholar] [CrossRef]
- Khalifa, L.; Shlezinger, M.; Beyth, S.; Houri-Haddad, Y.; Coppenhagen-Glazer, S.; Beyth, N.; Hazan, R. Phage therapy against Enterococcus faecalis in dental root canals. J. Oral Microbiol. 2016, 8, 32157. [Google Scholar] [CrossRef]
- Fong, S.A.; Drilling, A.; Morales, S.; Cornet, M.E.; Woodworth, B.A.; Fokkens, W.J.; Psaltis, A.J.; Vreugde, S.; Wormald, P.-J. Activity of bacteriophages in removing biofilms of Pseudomonas aeruginosa isolates from chronic rhinosinusitis patients. Front. Cell. Infect. Microbiol. 2017, 7, 418. [Google Scholar] [CrossRef]
- Szafrański, S.P.; Winkel, A.; Stiesch, M. The use of bacteriophages to biocontrol oral biofilms. J. Biotechnol. 2017, 250, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W.; Hughes, K.A.; Skillman, L.C.; Tait, K. The interaction of phage and biofilms. FEMS Microbiol. Lett. 2004, 232, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, L.; Brosh, Y.; Gelman, D.; Coppenhagen-Glazer, S.; Beyth, S.; Poradosu-Cohen, R.; Que, Y.A.; Beyth, N.; Hazan, R. Targeting Enterococcus faecalis biofilms with phage therapy. Appl Environ. Microbiol 2015, 81, 2696–2705. [Google Scholar] [CrossRef] [PubMed]
- Furie, B.; Furie, B.C. The molecular basis of blood coagulation. Cell 1988, 53, 505–518. [Google Scholar] [CrossRef]
- Houlihan, H.H.; Stokes, D.P.; Rybak, M.J. Pharmacodynamics of vancomycin and ampicillin alone and in combination with gentamicin once daily or thrice daily against Enterococcus faecalis in an in vitro infection model. J. Antimicrob. Chemother. 2000, 46, 79–86. [Google Scholar] [CrossRef]
- Kastrup, C.J.; Boedicker, J.Q.; Pomerantsev, A.P.; Moayeri, M.; Bian, Y.; Pompano, R.R.; Kline, T.R.; Sylvestre, P.; Shen, F.; Leppla, S.H. Spatial localization of bacteria controls coagulation of human blood by’quorum acting’. Nat. Chem. Biol. 2008, 4, 742–750. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hetta, H.F.; Rashed, Z.I.; Ramadan, Y.N.; Al-Kadmy, I.M.S.; Kassem, S.M.; Ata, H.S.; Nageeb, W.M. Phage Therapy, a Salvage Treatment for Multidrug-Resistant Bacteria Causing Infective Endocarditis. Biomedicines 2023, 11, 2860. https://doi.org/10.3390/biomedicines11102860
Hetta HF, Rashed ZI, Ramadan YN, Al-Kadmy IMS, Kassem SM, Ata HS, Nageeb WM. Phage Therapy, a Salvage Treatment for Multidrug-Resistant Bacteria Causing Infective Endocarditis. Biomedicines. 2023; 11(10):2860. https://doi.org/10.3390/biomedicines11102860
Chicago/Turabian StyleHetta, Helal F., Zainab I. Rashed, Yasmin N. Ramadan, Israa M. S. Al-Kadmy, Soheir M. Kassem, Hesham S. Ata, and Wedad M. Nageeb. 2023. "Phage Therapy, a Salvage Treatment for Multidrug-Resistant Bacteria Causing Infective Endocarditis" Biomedicines 11, no. 10: 2860. https://doi.org/10.3390/biomedicines11102860