Unraveling the Thread of Aphasia Rehabilitation: A Translational Cognitive Perspective
Abstract
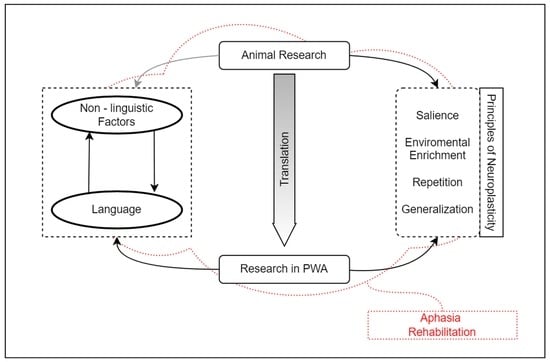
:1. Introduction
2. Neuroplasticity in Animals and Aphasia Research
3. Generalization, Environmental Enrichment, and Salience in Rehabilitation
4. Repetitio Est Mater Studiorum or “Repetition Influences Recovery”
5. Rehabilitation of Cognitive Functions and Its Reflection to Language
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Basso, A. Aphasia and Its Therapy; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Kasselimis, D.S.; Potagas, C. Language Disorders, Treatment and Remediation of James Wright. In Encyclopedia of the Social & Behavioral Sciences, 2nd ed.; Elsevier: Oxford, UK, 2015; pp. 329–336. [Google Scholar]
- Rijntjes, M.; Weiller, C.; Bormann, T.; Musso, M. The dual loop model: Its relation to language and other modalities. Front. Evol. Neurosci. 2012, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, E.B.; Descoteaux, M.; Petrides, M. Dissociating the white matter tracts connecting the temporo-parietal cortical region with frontal cortex using diffusion tractography. Sci. Rep. 2020, 10, 8186. [Google Scholar] [CrossRef]
- Kasselimis, D.; Angelopoulou, G.; Simos, P.; Petrides, M.; Peppas, C.; Velonakis, G.; Potagas, C. Working memory impairment in aphasia: The issue of stimulus modality. J. Neurolinguistics 2018, 48, 104–116. [Google Scholar] [CrossRef]
- Tettamanti, M.; Weniger, D. Broca’s area: A supramodal hierarchical processor? Cortex 2006, 42, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Hoen, M.; Pachot-Clouard, M.; Segebarth, C.; Dominey, P.F. When Broca experiences the Janus syndrome: An ER-fMRI study comparing sentence comprehension and cognitive sequence processing. Cortex 2006, 42, 605–623. [Google Scholar] [CrossRef] [PubMed]
- Schubotz, R.I.; Fiebach, C.J. Integrative models of Broca’s Area and the Ventral Premortor. Cortex 2006, 42, 461–463. [Google Scholar] [CrossRef]
- Fadiga, L.; Craighero, L.; Roy, A. Broca’s region: A speech area? In Broca’s Region; Grodzinsky, Y., Ed.; Oxford University Press: Oxford, UK, 2006; pp. 137–152. [Google Scholar]
- Kostopoulos, P.; Petrides, M. Selective memory retrieval of auditory what and auditory where involves the ventrolateral prefrontal cortex. Proc. Natl. Acad. Sci. USA 2016, 113, 1919–1924. [Google Scholar] [CrossRef]
- Champod, A.S.; Petrides, M. Dissociation within the frontoparietal network in verbal working memory: A parametric functional magnetic resonance imaging study. J. Neuro. Sci. 2010, 30, 3849–3866. [Google Scholar] [CrossRef]
- Champod, A.S.; Petrides, M. Dissociable Roles of the Posterior Parietal and the Prefrontal Cortex in Manipulation and Monitoring Processes. Proc. Natl. Acad. Sci. USA 2007, 104, 14837–14842. [Google Scholar] [CrossRef]
- Janelle, F.; Iorio-Morin, C.; D’amour, S.; Fortin, D. Superior Longitudinal Fasciculus: A Review of the Anatomical Descriptions with Functional Correlates. Front. Neurol. 2022, 13, 794618. [Google Scholar] [CrossRef]
- Petrides, M.; Pandya, D.N. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J. Comp. Neurol. 1988, 273, 52–66. [Google Scholar] [CrossRef]
- Petrides, M.; Pandya, D.N. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 2002, 16, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Ayala, J. Measurements of auditory-verbal STM span in aphasia: Effects of item, task, and lexical impairment. Brain Lang. 2004, 89, 463–483. [Google Scholar] [CrossRef] [PubMed]
- Minkina, I.; Salis, C.; Martin, N. Short-term and working memory deficits in aphasia: Current issues in theory, evidence, and treatment. J. Neuroling. 2018, 48, 1–3. [Google Scholar] [CrossRef]
- Fonseca, J.; Ferreira, J.J.; Martins, I.P. Cognitive performance in aphasia due to stroke: A systematic review. Int. J. Disabil. Hum. Dev. 2016, 16, 127–139. [Google Scholar] [CrossRef]
- Price, C.J. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012, 62, 816–847. [Google Scholar] [CrossRef]
- Baldo, J.V.; Dronkers, N.F. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology 2006, 20, 529. [Google Scholar] [CrossRef]
- Leff, A.P.; Schofield, T.M.; Crinion, J.T.; Seghier, M.L.; Grogan, A.; Green, D.W.; Price, C.J. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: Evidence from 210 patients with stroke. Brain 2009, 132, 3401–3410. [Google Scholar] [CrossRef]
- Chapados, C.; Petrides, M. Ventrolateral and dorsomedial frontal cortex lesions impair mnemonic context retrieval. Proc. R. Soc. B Nat. Environ. 2015, 282, 1801. [Google Scholar] [CrossRef]
- Kourtidou, E.; Kasselimis, D.; Angelopoulou, G.; Karavasilis, E.; Velonakis, G.; Kelekis, N.; Petrides, M. Specific disruption of the ventral anterior temporo-frontal network reveals key implications for language comprehension and cognition. Commun. Biol. 2022, 5, 1077. [Google Scholar] [CrossRef]
- Kiran, S.; Thompson, C.K. Neuroplasticity of Language Networks in Aphasia: Advances, Updates, and Future Challenges. Front. Neurol. 2019, 10, 295. [Google Scholar] [CrossRef]
- Keefe, K.A. Applying basic neuroscience to aphasia therapy: What the animals are tellingus. Am. J. Speech Lang. Pathol. 1995, 4, 88–93. [Google Scholar] [CrossRef]
- Turkstra, L.S.; Holland, A.L.; Bays, G.A. The neuroscience of recovery and rehabilitation:What have we learned from animal research? Arch. Phys. Med. Rehabil. 2003, 84, 604–612. [Google Scholar] [CrossRef]
- Raymer, A.M.; Holland, A.; Kendall, D.; Maher, L.M.; Martin, N.; Murray, L.; Gonzalez Rothi, L.J. Translational research in aphasia: From neuroscience to neurorehabilitation. J. Speech Lang. Hear. Res. 2007, 50, S259–S275. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, P.; Rönnbäck, A.; Bergström, S.A.; Söderström, I.; Olsson, T. Environmental enrichment reverses learning impairment in the Morris water maze after focal cerebral ischemia in rats. Eur. J. Neurosci. 2004, 19, 2288–2298. [Google Scholar] [CrossRef]
- Kaas, J. Evolution of Nervous Systems; Elsevier: New York, NY, USA, 2006. [Google Scholar]
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. of speech, language, and hearing research. J. Speech Lang. Hear. Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef] [PubMed]
- Rockel, A.J.; Hiorns, R.W.; Powell, T.P. The basic uniformity in structure of the neocortex. Brain 1980, 103, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Buonomano, D.V.; Merzenich, M.M. Cortical plasticity: From synapses to maps. Ann. Rev. Neurosci. 1998, 21, 149–186. [Google Scholar] [CrossRef] [PubMed]
- Farokhi-Sisakht, F.; Farhoudi, M.; Sadigh-Eteghad, S.; Mahmoudi, J.; Mohaddes, G. Cognitive Rehabilitation Improves Ischemic Stroke-Induced Cognitive Impairment: Role of Growth Factors. J. Stroke Cerebrovasc. Dis. 2019, 28, 104299. [Google Scholar] [CrossRef]
- Kleim, J.A. Neural plasticity and neurorehabilitation: Teaching the new brain old tricks. J. Commun. Disord. 2011, 44, 521–528. [Google Scholar] [CrossRef]
- Valenzuela, M.J.; Jones, M.; Wen, W.; Rae, C.; Graham, S.; Shnier, R.; Sachdev, P. Memory training alters hippocampal neurochemistry in healthy elderly. Neuroreport 2003, 14, 1333–1337. [Google Scholar] [CrossRef]
- Berlucchi, G. Brain plasticity and cognitive neurorehabilitation. Neuropsychol. Rehabil. 2011, 21, 560–578. [Google Scholar] [CrossRef]
- Thompson, C.K. Neuroplasticity: Evidence from aphasia. J. Commun. Disord. 2000, 33, 357–366. [Google Scholar] [CrossRef]
- Fridriksson, J. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. J. Neurosci. 2010, 30, 11558–11564. [Google Scholar] [CrossRef] [PubMed]
- Staudt, M.; Lidzba, K.; Grodd, W.; Wildgruber, D.; Erb, M.; Krägeloh-Mann, I. Right-hemispheric organization of language following early left-sided brain lesions: Functional MRI topography. NeuroImage 2002, 16, 954–967. [Google Scholar] [CrossRef]
- Martin, P.I.; Naeser, M.A.; Ho, M.; Treglia, E.; Kaplan, E.; Baker, E.H.; Pascual-Leone, A. Research with transcranial magnetic stimulation in the treatment of aphasia. Curr. Neurol. Neurosci. Rep. 2009, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.K.; Riley, E.A.; den Ouden, D.B.; Meltzer-Asscher, A.; Lukic, S. Training verb argument structure production in agrammatic aphasia: Behavioral and neural recovery patterns. Cortex 2013, 49, 2358–2376. [Google Scholar] [CrossRef]
- Fridriksson, J.; Morrow-Odom, L.; Moser, D.; Fridriksson, A.; Baylis, G. Neural recruitment associated with anomia treatment in aphasia. Neuroimage 2006, 32, 1403–1412. [Google Scholar] [CrossRef]
- Kiran, S.; Meier, E.L.; Kapse, K.J.; Glynn, P.A. Changes in task-based effective connectivity in language networks following rehabilitation in post-stroke patients with aphasia. Front. Hum. Neurosci. 2015, 9, 316. [Google Scholar] [CrossRef]
- Crosson, B.; Rodriguez, A.D.; Copland, D.; Fridriksson, J.; Krishnamurthy, L.C.; Meinzer, M.; Raymer, A.M.; Krishnamurthy, V.; Leff, A.P. Neuroplasticity and aphasia treatments: New approaches for an old problem. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1147–1155. [Google Scholar] [CrossRef]
- Schlaug, G.; Marchina, S.; Norton, A. Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Ann. N. Y. Acad. Sci. 2009, 1169, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Allendorfer, J.B.; Storrs, J.M.; Szaflarski, J.P. Changes in white matter integrity follow excitatory rTMS treatment of post-stroke aphasia. Restor. Neurol. Neurosci. 2012, 30, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R. Cellular Mechanisms of Learning and the Biological Basis of Individuality. In Principles of Neuroscience; Kandel, E.R., Schwartz, J.H., Eds.; Elsevier Science Publishers: New York, NY, USA, 1985. [Google Scholar]
- Jenkins, W.M.; Merzenich, M.M.; Recanzone, G. Neocortical representational dynamics in adult primates: Implications for neuropsychology. Neuropsychologia 1990, 28, 573–584. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Li, L.; Zhang, Y.; Yang, M.; Liang, S.; Li, L.; Dai, Y.; Chen, L.; Jia, W.; et al. Enhanced Medial Prefrontal Cortex and Hippocampal Activity Improves Memory Generalization in APP/PS1 Mice: A Multimodal Animal MRI Study. Front. Cell. Neurosci. 2022, 16, 848967. [Google Scholar] [CrossRef]
- Kolb, B. Brain Plasticity and Behavior; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1995. [Google Scholar]
- York, A.; Breedlove, S.M.; Diamond, M.A. Increase in granule cell neurogenesis following exposure to enriched environments. Neurosci. Abstr. 1989, 15, 602. [Google Scholar]
- Zentall, T.R. Effect of Environmental Enrichment on the Brain and on Learning and Cognition by Animals. Animals 2021, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- Hamm, R.J.; Temple, M.D.; Pike, B.R.; O’Dell, D.M.; Buck, D.L.; Lyeth, B.G. Working memory deficits following traumatic brain injury in the rat. J. Neurotrauma 1996, 13, 317–323. [Google Scholar] [CrossRef]
- Vasn Dellen, A.; Blakemore, C.; Deacon, R.; York, D.; Hannan, A.J. Delaying the onset of Huntington’s in mice. Nature 2000, 404, 721–722. [Google Scholar] [CrossRef]
- Han, P.P.; Han, Y.; Shen, X.Y.; Gao, Z.K.; Bi, X. Enriched environment-induced neuroplasticity in ischemic stroke and its underlying mechanisms. Front. Cell Neurosci. 2023, 17, 1210361. [Google Scholar] [CrossRef]
- Weinberger, N.M. Specific long-term memory traces in primary auditory cortex. Nat. Rev. Neurosci. 2004, 5, 279–290. [Google Scholar] [CrossRef]
- Kiran, S.; Thompson, C.K. The role of semantic complexity in treatment of naming deficits: Training semantic categories in fluent aphasia by controlling exemplar typicality. J. Speech Lang. Hear. Res. 2003, 46, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Nickels, L. Therapy for naming disorders: Revisiting, revising, and reviewing. Aphasiology 2002, 16, 935–979. [Google Scholar] [CrossRef]
- Hillis, A.E.; Beh, Y.Y.; Sebastian, R.; Breining, B.; Tippett, D.C.; Wright, A.; Saxena, S.; Rorden, C.; Bonilha, L.; Basilakos, A.; et al. Predicting recovery in acute poststroke aphasia. Ann. Neurol. 2018, 83, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Howard, D. Cognitive Neuropsychology and Aphasia Therapy: The Case of Word Retrieval. In Acquired Neurogenic Communication Disorders: A Clinical Perspective; Papathanasiou, I., Ed.; Whurr: London, UK, 2000. [Google Scholar]
- Thompson, C.K.; Shapiro, L.P. Complexity in treatment of syntactic deficits. Am. J. Speech Lang. Pathol. 2007, 16, 30–42. [Google Scholar] [CrossRef]
- Janssen, H.; Ada, L.; Middleton, S.; Pollack, M.; Nilsson, M.; Churilov, L.; Blennerhassett, J.; Faux, S.; New, P.; McCluskey, A.; et al. Altering the rehabilitation environment to improve stroke survivor activity: A Phase II trial. Int. J. Stroke 2022, 17, 299–307. [Google Scholar] [CrossRef]
- Kleim, J.A.; Bruneau, R.; VandenBerg, P.; MacDonald, E.; Mulrooney, R.; Pocock, D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol. Res. 2003, 25, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Kilgard, M.P.; Merzenich, M.M. Cortical map reorganization enabled by nucleus basalis activity. Science 1998, 279, 1714–1718. [Google Scholar] [CrossRef]
- Monfils, M.H.; Teskey, G.C. Skilled-learning-induced potentiation in rat sensorimotor cortex: A transient form of behavioural long-term potentiation. Neuroscience 2004, 125, 329–336. [Google Scholar] [CrossRef]
- Xing, Y.; Bai, Y. A Review of Exercise-Induced Neuroplasticity in Ischemic Stroke: Pathology and Mechanisms. Mol. Neurobiol. 2020, 57, 4218–4231. [Google Scholar] [CrossRef]
- Koganemaru, S.; Sawamoto, N.; Aso, T.; Sagara, A.; Ikkaku, T.; Shimada, K.; Kanematsu, M.; Takahashi, R.; Domen, K.; Fu-kuyama, H.; et al. Task-specific brain reorganization in motor recovery induced by a hybrid-rehabilitation combining training with brain stimulation after stroke. Neurosci. Res. 2015, 92, 29–38. [Google Scholar] [CrossRef]
- Luke, L.M.; Allred, R.P.; Jones, T.A. Unilateral ischemic sensorimotor cortical damage induces contralesional synaptogenesis and enhances skilled reaching with the ipsilateral forelimb in adult male rats. Synapse 2004, 54, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, D.A.; James, D.C.; Schallert, T. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J. Neurosci. 1996, 16, 4776–4786. [Google Scholar] [CrossRef] [PubMed]
- Cherney, L.R.; Patterson, J.P.; Raymer, A.M. Intensity of aphasia therapy: Evidence and efficacy. Curr. Neurol. Neurosci. Rep. 2011, 11, 560–569. [Google Scholar] [CrossRef]
- Sage, K.; Snell, C.; Lambon Ralph, M.A. How intensive does anomia therapy for people with aphasia need to be? Neuropsychol. Rehabil. 2011, 21, 26–41. [Google Scholar] [CrossRef]
- Pulvermüller, F.; Neininger, B.; Elbert, T.; Mohr, B.; Rockstroh, B.; Koebbel, P.; Taub, E. Constraint-induced therapy of chronic aphasia after stroke. Stroke 2001, 32, 1621–1626. [Google Scholar] [CrossRef]
- Bhogal, S.K.; Teasell, R.; Speechley, M. Intensity of aphasia therapy, impact on recovery. Stroke 2003, 34, 987–993. [Google Scholar] [CrossRef]
- Meinzer, M.; Flaisch, T.; Breitenstein, C.; Wienbruch, C.; Elbert, T.; Rockstroh, B. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. NeuroImage 2008, 39, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Badre, D.; Wagner, A.D. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 2007, 45, 2883–2901. [Google Scholar] [CrossRef]
- Tremblay, P.; Dick, A.S. Broca and Wernicke are dead, or moving past the classic model of language neurobiology. Brain Lang. 2016, 162, 60–71. [Google Scholar] [CrossRef]
- Rapp, B.; Caplan, D.; Edwards, S.; Visch-Brink, E.; Thompson, C.K. Neuroimaging in aphasia treatment research: Issues of experimental design for relating cognitive to neural changes. Neuroimage 2013, 73, 200–207. [Google Scholar] [CrossRef]
- Gainotti, G. Old and recent approaches to the problem of non-verbal conceptual disorders in aphasic patients. Cortex 2014, 53, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.M.; Martin, R.C.; Martin, N. Relations between Short-term Memory Deficits, Semantic Processing, and Executive Function. Aphasiology 2012, 26, 428–461. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.J.; Quitz, A. Verbal and nonverbal memory impairment in aphasia. J. Neurol. 2012, 259, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.L.; Keeton, R.J.; Karcher, L. Treating attention in mild aphasia: Evaluation of attention process training-II. J. Commun. Disord. 2006, 39, 37–61. [Google Scholar] [CrossRef]
- Lesniak, M.; Bak, T.; Czepiel, W.; Seniow, J.; Czlonkowska, A. Frequency and prognostic value of cognitive disorders in stroke patients. Dement. Geriatr. Cogn. Disord. 2008, 26, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Purdy, M. Executive function ability in persons with aphasia. Aphasiology 2002, 16, 549–557. [Google Scholar] [CrossRef]
- Sekine, K.; Rose, M.L. The relationship of aphasia type and gesture production in people with aphasia. Am. J. Speech Lang. Pathol. 2013, 22, 662–672. [Google Scholar] [CrossRef]
- Murray, L.L.; Holland, A.L.; Beeson, P.M. Auditory processing in individuals with mild aphasia: A study of resource allocation. J. Speech Lang. Hear. Res. 1997, 40, 792–808. [Google Scholar] [CrossRef]
- Helm-Estabrooks, N.; Connor, L.T.; Albert, M.L. Training attention to improve auditory comprehension in aphasia. Brain Lang. 2000, 74, 469–472. [Google Scholar] [CrossRef]
- Marrelec, G.; Bellec, P.; Krainik, A.; Duffau, H.; Pélégrini-Issac, M.; Lehéricy, S.; Benali, H.; Doyon, J. Regions, systems, and the brain: Hierarchical measures of functional integration in fMRI. Med. Image Anal. 2008, 12, 484–496. [Google Scholar] [CrossRef]
- Kasselimis, D.S.; Simos, P.G.; Economou, A.; Peppas, C.; Evdokimidis, I.; Potagas, C. Are memory deficits dependent on the presence of aphasia in left brain damaged patients? Neuropsychologia 2013, 51, 1773–1776. [Google Scholar] [CrossRef] [PubMed]
- Koenig-Bruhin, M.; Studer-Eichenberger, F. Therapy of short-term memory disorders in fluent aphasia: A single case study. Aphasiology 2007, 21, 448–458. [Google Scholar] [CrossRef]
- Friedmann, N.; Gvion, A. Sentence comprehension and working memory limitation in aphasia: A dissociation between semantic-syntactic and phonological reactivation. Brain Lang. 2003, 86, 23–39. [Google Scholar] [CrossRef]
- Gilmore, N.; Meier, E.L.; Johnson, J.P.; Kiran, S. Nonlinguistic Cognitive Factors Predict Treatment-Induced Recovery in Chronic Poststroke Aphasia. Arch. Phys. Med. Rehabil. 2019, 100, 1251–1258. [Google Scholar] [CrossRef]
- Blumstein, S.E.; Amso, D. Dynamic Functional Organization of Language: Insights from Functional Neuroimaging. Perspect Psychol Sci. 2013, 8, 44–48. [Google Scholar] [CrossRef]
- Watila, M.M.; Balarabe, S.A. Factors predicting post-stroke aphasia recovery. J. Neurol. Sci. 2015, 12, 352. [Google Scholar] [CrossRef]
- Lazar, R.M.; Antoniello, D. Variability in recovery from aphasia. Curr. Neurol. Neurosci. Rep. 2008, 8, 497–502. [Google Scholar] [CrossRef]
- Laska, A.C.; Hellblom, A.; Murray, V.; Kahan, T.; Von Arbin, M. Aphasia in acute stroke and relation to outcome. J. Intern. Med. 2001, 249, 413–422. [Google Scholar] [CrossRef]
- Inatomi, Y.; Yonehara, T.; Omiya, S.; Hashimoto, Y.; Hirano, T.; Uchino, M. Aphasia during the acute phase in ischemic stroke. Cerebrovasc. Dis. 2008, 25, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, M.; Vista, M.; Pardossi, L.; Avila, L.; Bianchi, F.; Moretti, P. Spontaneous evolution of aphasia after ischaemic stroke. Aphasiology 2007, 6, 387–396. [Google Scholar] [CrossRef]
- Maas, M.B.; Lev, M.H.; Ay, H.; Singhal, A.B.; Greer, D.M.; Smith, W.S.; Harris, G.J.; Halpern, E.F.; Koroshetz, W.J.; Furie, K.L. The prognosis for aphasia in stroke. J. Stroke Cerebrovasc. Dis. 2012, 21, 350–357. [Google Scholar] [CrossRef]
- Kasselimis, D.; Papageorgiou, G.; Angelopoulou, G.; Tsolakopoulos, D.; Potagas, C. Translational Neuroscience of Aphasia and Adult Language Rehabilitation. In Neuroscience of Speech and Language Disorders; Argyropoulos, G., Ed.; Contemporary Clinical Neuroscience Series; Springer: Berlin, Germany, 2020; pp. 5–20. [Google Scholar]
- Boyle, M. Semantic feature analysis treatment for aphasic word retrieval impairments: What’s in a name? Top. Stroke Rehabil. 2010, 17, 411–412. [Google Scholar] [CrossRef]
- Kendall, D.L.; Oelke, M.; Brookshire, C.E.; Nadeau, S.E. The influence of phonomotor treatment on word retrieval abilities in 26 individuals with chronic aphasia: An open trial. J. Speech Language Hear. Res. 2015, 58, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.L.; Towler, S.; Garcia, A.; Park, H.; Sudhyadhom, A.; Harnish, S.; McGregor, K.M.; Zlatar, Z.; Reilly, J.J.; Rosenbek, J.C.; et al. A behavioral manipulation engages right frontal cortex during aphasia therapy. Neurorehab. Neural Rep. 2014, 28, 545–553. [Google Scholar] [CrossRef]
- Dromerick, A.W.; Edwardson, M.A.; Edwards, D.F.; Giannetti, M.L.; Barth, J.; Brady, K.P.; Chan, E.; Tan, M.T.; Tamboli, I.; Chia, R.; et al. Critical periods after stroke study: Translating animal stroke recovery experiments into a clinical trial. Front. Hum. Neurosci. 2015, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Dignam, J.; Copland, D.; McKinnon, E.; Burfein, P.; O’Brien, K.; Farrell, A.; Rodriguez, A.D. Intensive Versus Distributed Aphasia Therapy: A Nonrandomized, Parallel-Group, Dosage-Controlled Study. Stroke 2015, 46, 2206–2211. [Google Scholar] [CrossRef]
- Woodlee, M.T.; Schallert, T. The interplay between behavior and neurodegeneration in rat models of Parkinson’s disease and stroke. Restor. Neurol. Neurosci. 2004, 22, 153–161. [Google Scholar] [PubMed]
- Kapoor, A. Repetitive transcranial magnetic stimulation therapy for post-stroke non-fluent aphasia: A critical review. Top. Stroke Rehabil. 2017, 24, 547–553. [Google Scholar] [CrossRef]
- Shah, P.P.; Szaflarski, J.P.; Allendorfer, J.; Hamilton, R.H. Induction of neuroplasticity and recovery in post-stroke aphasia by non-invasive brain stimulation. Front. Hum. Neurosci. 2013, 7, 888. [Google Scholar] [CrossRef]
- Dmochowski, J.P.; Datta, A.; Huang, Y.; Richardson, J.D.; Bikson, M.; Fridriksson, J.; Parra, L.C. Targeted transcranial direct current stimulation for rehabilitation after stroke. Neuroimage 2013, 75, 12–19. [Google Scholar] [CrossRef]
- Lourbopoulos, A.; Mourouzis, I.; Xinaris, C.; Zerva, N.; Filippakis, K.; Pavlopoulos, A.; Pantos, C. Translational Block in Stroke: A Constructive and “Out-of-the-Box” Reappraisal. Front. Neurosci. 2021, 15, 652403. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papageorgiou, G.; Kasselimis, D.; Laskaris, N.; Potagas, C. Unraveling the Thread of Aphasia Rehabilitation: A Translational Cognitive Perspective. Biomedicines 2023, 11, 2856. https://doi.org/10.3390/biomedicines11102856
Papageorgiou G, Kasselimis D, Laskaris N, Potagas C. Unraveling the Thread of Aphasia Rehabilitation: A Translational Cognitive Perspective. Biomedicines. 2023; 11(10):2856. https://doi.org/10.3390/biomedicines11102856
Chicago/Turabian StylePapageorgiou, Georgios, Dimitrios Kasselimis, Nikolaos Laskaris, and Constantin Potagas. 2023. "Unraveling the Thread of Aphasia Rehabilitation: A Translational Cognitive Perspective" Biomedicines 11, no. 10: 2856. https://doi.org/10.3390/biomedicines11102856