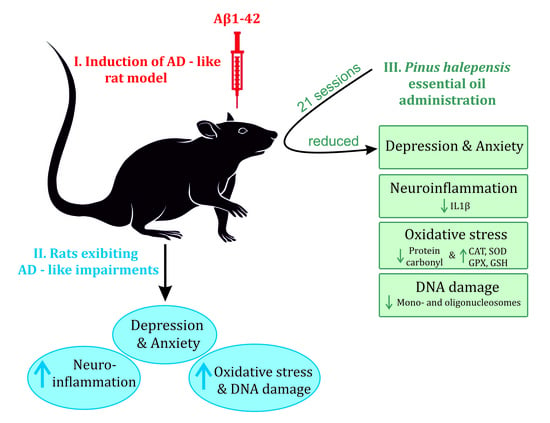
Pinus halepensis Essential Oil Ameliorates Aβ1-42-Induced Brain Injury by Diminishing Anxiety, Oxidative Stress, and Neuroinflammation in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production and Analysis of Essential Oil
2.2. Animals
2.3. Experimental Design
2.4. Drug Administration
2.4.1. Intracerebroventricular (i.c.v.) Route
2.4.2. Intraperitoneal (i.p.) Route
2.4.3. Inhalation Sessions
2.5. Behavioral Analysis
2.5.1. Elevated plus Maze Test
2.5.2. Forced Swimming Test
2.6. Animal Euthanasia and Tissue Collection
2.7. Biochemical Parameters Assay
2.7.1. Protein Extraction
2.7.2. Catalase Activity Assessment
2.7.3. Superoxide Dismutase Activity Assessment
2.7.4. Glutathione Peroxidase Activity Assessment
2.7.5. The Total Content of Reduced Glutathione Assessment
2.7.6. Protein Carbonyl Level Assessment
2.7.7. Malondialdehyde Level Assessment
2.8. DNA Fragmentation Assay
2.9. RNA Isolation and Amygdala Real-Time Quantitative PCR (qRT-PCR)
2.10. Statistical Analysis
3. Results and Discussion
3.1. The Effects of PNO on Anxious–Depressive-Like Behaviors
3.2. The Effects of PNO on ARC mRNA Level
3.3. The Effects of PNO on Neuroinflammation—AD-Related
3.4. The Effects of PNO on Oxidative Stress—AD-Related
3.5. The Effects of PNO on Cell Death—AD-Related
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Lantero Rodriguez, J.; Karikari, T.K.; Suárez-Calvet, M.; Troakes, C.; King, A.; Emersic, A.; Aarsland, D.; Hye, A.; Zetterberg, H.; Blennow, K.; et al. Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020, 140, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.A.; Potashkin, J.A. The Impact of Disease Comorbidities in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 631770. [Google Scholar] [CrossRef]
- Fiandaca, M.S.; Mapstone, M.E.; Cheema, A.K.; Federoff, H.J. The critical need for defining preclinical biomarkers in Alzheimer’s disease. Alzheimers Dement. 2014, 10, S196–S212. [Google Scholar] [CrossRef] [PubMed]
- Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, A.M.; Winblad, B.; Jönsson, L.; Liu, Z.; Prince, M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- El-Hayek, Y.H.; Wiley, R.E.; Khoury, C.P.; Daya, R.P.; Ballard, C.; Evans, A.R.; Karran, M.; Molinuevo, J.L.; Norton, M.; Atri, A. Tip of the Iceberg: Assessing the Global Socioeconomic Costs of Alzheimer’s Disease and Related Dementias and Strategic Implications for Stakeholders. J. Alzheimers Dis. 2019, 70, 323. [Google Scholar] [CrossRef]
- Pena, D.; Suescun, J.; Schiess, M.; Ellmore, T.M.; Giancardo, L. Toward a Multimodal Computer-Aided Diagnostic Tool for Alzheimer’s Disease Conversion. Front. Neurosci. 2022, 15, 1644. [Google Scholar] [CrossRef]
- Ritchie, C.W.; Mason, S.E.; McShane, R. Diagnostic tests for Alzheimer’s disease: Rationale, methodology, and challenges. Int. J. Alzheimers Dis. 2010, 2010, 972685. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.J.; Kim, D.W.; Kim, S.Y.; Park, S.; Park, C.B. Clinically accurate diagnosis of Alzheimer’s disease via multiplexed sensing of core biomarkers in human plasma. Nat. Commun. 2020, 11, 119. [Google Scholar] [CrossRef]
- Pressman, P.; Rabinovici, G. Alzheimer’s Disease. In Encyclopedia of the Neurological Sciences; Aminoff, M., Daroff, R., Eds.; Academic Press Inc.: Oxford, UK, 2014; pp. 122–127. [Google Scholar]
- Mühlbacher, A.; Johnson, F.R.; Yang, J.C.; Happich, M.; Belger, M. Do You Want to Hear the Bad News? The Value of Diagnostic Tests for Alzheimer’s Disease. Value Health 2016, 19, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Peter, J.; Klöppel, S. Alzheimer’s Disease. In Brain Mapping; Toga, A., Ed.; Academic Press Inc.: Waltham, MA, USA, 2015; pp. 647–651. [Google Scholar]
- Turner, R.S.; Stubbs, T.; Davies, D.A.; Albensi, B.C. Potential New Approaches for Diagnosis of Alzheimer’s Disease and Related Dementias. Front. Neurol. 2020, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Hsu, J.L.; Lin, K.J.; Liu, H.L.; Wey, S.P.; Hsiao, I.T.; Weiner, M.; Aisen, P.; Petersen, R.; Jack, C.R.; et al. Characteristic patterns of inter- and intra-hemispheric metabolic connectivity in patients with stable and progressive mild cognitive impairment and Alzheimer’s disease. Sci. Rep. 2018, 8, 13807. [Google Scholar] [CrossRef] [PubMed]
- Kiselica, A.M. Empirically defining the preclinical stages of the Alzheimer’s continuum in the Alzheimer’s Disease Neuroimaging Initiative. Psychogeriatrics 2021, 21, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B. The Emergence of a New Conceptual Framework for Alzheimer’s Disease. J. Alzheimers Dis. 2018, 62, 1059–1066. [Google Scholar] [CrossRef]
- Aisen, P.S.; Cummings, J.; Jack, C.R.; Morris, J.C.; Sperling, R.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimers Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimers Dis. 2021, 8, 371–386. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S.; Trzepacz, P.; Amatniek, J.; Cedarbaum, J.; Brashear, R.; Miller, D.S. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011, 7, 532–539. [Google Scholar] [CrossRef]
- Eikelboom, W.S.; van den Berg, E.; Singleton, E.H.; Baart, S.J.; Coesmans, M.; Leeuwis, A.E.; Teunissen, C.E.; van Berckel, B.N.M.; Pijnenburg, Y.A.L.; Scheltens, P.; et al. Neuropsychiatric and Cognitive Symptoms Across the Alzheimer Disease Clinical Spectrum. Neurology 2021, 97, e1276–e1287. [Google Scholar] [CrossRef]
- Li, X.L.; Hu, N.; Tan, M.S.; Yu, J.T.; Tan, L. Behavioral and psychological symptoms in Alzheimer’s disease. Biomed. Res. Int. 2014, 2014, 927804. [Google Scholar] [CrossRef]
- Nowrangi, M.A.; Lyketsos, C.G.; Rosenberg, P.B. Principles and management of neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimers Res. Ther. 2015, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Lanctôt, K.L.; Amatniek, J.; Ancoli-Israel, S.; Arnold, S.E.; Ballard, C.; Cohen-Mansfield, J.; Ismail, Z.; Lyketsos, C.; Miller, D.S.; Musiek, E.; et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: New treatment paradigms. Alzheimers Dement. 2017, 3, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Azocar, I.; Livingston, G.; Huntley, J. The Association Between Impaired Awareness and Depression, Anxiety, and Apathy in Mild to Moderate Alzheimer’s Disease: A Systematic Review. Front. Psychiatry 2021, 12, 633081. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.K.Y.; Chan, W.C.; Spector, A.; Wong, G.H.Y. Prevalence of depression, anxiety, and apathy symptoms across dementia stages: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2021, 36, 1330–1344. [Google Scholar] [CrossRef] [PubMed]
- Cantón-Habas, V.; Rich-Ruiz, M.; Romero-Saldaña, M.; Carrera-González, M.D.P. Depression as a Risk Factor for Dementia and Alzheimer’s Disease. Biomedicines 2020, 8, 457. [Google Scholar] [CrossRef]
- Becker, E.; Orellana Rios, C.L.; Lahmann, C.; Rücker, G.; Bauer, J.; Boeker, M. Anxiety as a risk factor of Alzheimer’s disease and vascular dementia. Br. J. Psychiatry 2018, 213, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Dafsari, F.S.; Jessen, F. Depression-an underrecognized target for prevention of dementia in Alzheimer’s disease. Transl. Psychiatry 2020, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Vale, N. Antidepressants in Alzheimer’s Disease: A Focus on the Role of Mirtazapine. Pharmaceuticals 2021, 14, 930. [Google Scholar] [CrossRef]
- Mendez, M.F. The Relationship Between Anxiety and Alzheimer’s Disease. J. Alzheimers Dis. 2021, 5, 171–177. [Google Scholar] [CrossRef]
- Burke, A.D.; Goldfarb, D.; Bollam, P.; Khokher, S. Diagnosing and Treating Depression in Patients with Alzheimer’s Disease. Neurol. Ther. 2019, 8, 325–350. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, X.; Wei, Q.; Wang, K.; Tian, Y. Differences in Cerebral Structure Associated With Depressive Symptoms in the Elderly With Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 107. [Google Scholar] [CrossRef]
- Lebedeva, A.; Westman, E.; Lebedev, A.V.; Li, X.; Winblad, B.; Simmons, A.; Wahlund, L.O.; Aarsland, D. Structural brain changes associated with depressive symptoms in the elderly with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2014, 85, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dang, M.; Zhang, Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: A systematic review of symptom-general and specific lesion patterns. Mol. Neurodegener. 2021, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Ortner, M.; Pasquini, L.; Barat, M.; Alexopoulos, P.; Grimmer, T.; Förster, S.; Diehl-Schmid, J.; Kurz, A.; Förstl, H.; Zimmer, C.; et al. Progressively Disrupted Intrinsic Functional Connectivity of Basolateral Amygdala in Very Early Alzheimer’s Disease. Front. Neurol. 2016, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.R.; Bergh, S.; Engedal, K.; Kirkevold, M.; Kirkevold, Ø. Anxiety, Anxiety Symptoms, and Their Correlates in Persons with Dementia in Norwegian Nursing Homes: A Cause for Concern. Dement. Geriatr. Cogn. Disord. 2017, 43, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef]
- Torres, K.C.; Lima, G.S.; Fiamoncini, C.M.I.; Rezende, V.B.; Pereira, P.A.; Bicalho, M.A.; Moraes, E.N.; Romano-Silva, M.A. Increased frequency of cluster of differentiation 14 (CD14+) monocytes expressing interleukin 1 beta (IL-1β) in Alzheimer’s disease patients and intermediate levels in late-onset depression patients. Int. J. Geriatr. Psychiatry 2014, 29, 137–143. [Google Scholar] [CrossRef]
- Polak-Szabela, A.; Dziembowska, I.; Bracha, M.; Pedrycz-Wieczorska, A.; Kedziora-Kornatowska, K.; Kozakiewicz, M. The Analysis of Oxidative Stress Markers May Increase the Accuracy of the Differential Diagnosis of Alzheimer’s Disease with and without Depression. Clin. Interv. Aging 2021, 16, 1105–1117. [Google Scholar] [CrossRef]
- Colucci-D’amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- Borroni, B.; Grassi, M.; Archetti, S.; Costanzi, C.; Bianchi, M.; Caimi, L.; Caltagirone, C.; Di Luca, M.; Padovani, A. BDNF genetic variations increase the risk of Alzheimer’s disease-related depression. J. Alzheimers. Dis. 2009, 18, 867–875. [Google Scholar] [CrossRef]
- Morgese, M.G.; Trabace, L. Monoaminergic System Modulation in Depression and Alzheimer’s Disease: A New Standpoint? Front. Pharmacol. 2019, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Babulal, G.M.; Roe, C.M.; Stout, S.H.; Rajasekar, G.; Wisch, J.K.; Benzinger, T.L.S.; Morris, J.C.; Ances, B.M. Depression is Associated with Tau and Not Amyloid Positron Emission Tomography in Cognitively Normal Adults. J. Alzheimers Dis. 2020, 74, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Rapp, M.A.; Schnaider-Beeri, M.; Purohit, D.P.; Perl, D.P.; Haroutunian, V.; Sano, M. Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression. Am. J. Geriatr. Psychiatry 2008, 16, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Stomrud, E.; Lindberg, O.; Westman, E.; Johansson, P.M.; van Westen, D.; Mattsson, N.; Hansson, O. Apathy and anxiety are early markers of Alzheimer’s disease. Neurobiol. Aging 2020, 85, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, R.H.; Lim, Y.Y.; Neumeister, A.; Ames, D.; Ellis, K.A.; Harrington, K.; Lautenschlager, N.T.; Restrepo, C.; Martins, R.N.; Masters, C.L.; et al. Amyloid-β, anxiety, and cognitive decline in preclinical Alzheimer disease: A multicenter, prospective cohort study. JAMA Psychiatry 2015, 72, 284–291. [Google Scholar] [CrossRef]
- Ehrenberg, A.J.; Suemoto, C.K.; de Paula França Resende, E.; Petersen, C.; Leite, R.E.P.; Rodriguez, R.D.; Ferretti-Rebustini, R.E.d.L.; You, M.; Oh, J.; Nitrini, R.; et al. Neuropathologic Correlates of Psychiatric Symptoms in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 66, 115–126. [Google Scholar] [CrossRef]
- Barbe, C.; Morrone, I.; Wolak-Thierry, A.; Dramé, M.; Jolly, D.; Novella, J.L.; Mahmoudi, R. Impact of functional alterations on quality of life in patients with Alzheimer’s disease. Aging Ment. Health 2017, 21, 571–576. [Google Scholar] [CrossRef]
- Goodarzi, Z.; Samii, L.; Azeem, F.; Sekhon, R.; Crites, S.; Pringsheim, T.; Smith, E.E.; Ismail, Z.; Holroyd-Leduc, J. Detection of anxiety symptoms in persons with dementia: A systematic review. Alzheimers Dement. 2019, 11, 340. [Google Scholar] [CrossRef]
- Goodarzi, Z.S.; Mele, B.S.; Roberts, D.J.; Holroyd-Leduc, J. Depression Case Finding in Individuals with Dementia: A Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2017, 65, 937–948. [Google Scholar] [CrossRef]
- Agüera-Ortiz, L.; García-Ramos, R.; Grandas Pérez, F.J.; López-Álvarez, J.; Montes Rodríguez, J.M.; Olazarán Rodríguez, F.J.; Olivera Pueyo, J.; Pelegrin Valero, C.; Porta-Etessam, J. Depression in Alzheimer’s Disease: A Delphi Consensus on Etiology, Risk Factors, and Clinical Management. Front. Psychiatry 2021, 12, 638651. [Google Scholar] [CrossRef]
- Cummings, J. New approaches to symptomatic treatments for Alzheimer’s disease. Mol. Neurodegener. 2021, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Defrancesco, M.; Marksteiner, J.; Wolfgang Fleischhacker, W.; Blasko, I. Use of Benzodiazepines in Alzheimer’s Disease: A Systematic Review of Literature. Int. J. Neuropsychopharmacol. 2015, 18, pyv055. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, M.; La Montagna, M.; D’Urso, F.; Piccininni, C.; Sardone, R.; Dibello, V.; Giannelli, G.; Solfrizzi, V.; Greco, A.; Daniele, A.; et al. Pharmacotherapy for the treatment of depression in patients with alzheimer’s disease: A treatment-resistant depressive disorder. Expert Opin. Pharmacother. 2018, 19, 823–842. [Google Scholar] [CrossRef]
- Phan, S.V.; Osae, S.; Morgan, J.C.; Inyang, M.; Fagan, S.C. Neuropsychiatric Symptoms in Dementia: Considerations for Pharmacotherapy in the USA. Drugs R D 2019, 19, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Fajemiroye, J.O.; da Silva, D.M.; de Oliveira, D.R.; Costa, E.A. Treatment of anxiety and depression: Medicinal plants in retrospect. Fundam. Clin. Pharmacol. 2016, 30, 198–215. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Therapeutic Effects of Phytochemicals and Medicinal Herbs on Depression. Biomed. Res. Int. 2017, 2017, 6596241. [Google Scholar] [CrossRef]
- Sarris, J.; McIntyre, E.; Camfield, D.A. Plant-based medicines for anxiety disorders, part 2: A review of clinical studies with supporting preclinical evidence. CNS Drugs 2013, 27, 301–319. [Google Scholar] [CrossRef]
- Lin, L.; Duan, R.; Yang, Q.; Li, T.; Zhou, H.; Hou, J.; Zhou, H. Effect of aromatherapy in patients with Alzheimer’s disease: A randomised controlled clinical trial. Res. Sq. 2022, 1–11. [Google Scholar] [CrossRef]
- Emami, S.A.; Shahani, A.; Hassanzadeh Khayyat, M. Antioxidant activity of leaves and fruits of cultivated conifers in iran. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 113–117. [Google Scholar] [CrossRef]
- Dhibi, M.; Issaoui, M.; Brahmi, F.; Mechri, B.; Mnari, A.; Cheraif, I.; Skhiri, F.; Gazzah, N.; Hammami, M. Nutritional quality of fresh and heated Aleppo pine (Pinus halepensis Mill.) seed oil: Trans-fatty acid isomers profiles and antioxidant properties. J. Food Sci. Technol. 2014, 51, 1442–1452. [Google Scholar] [CrossRef] [Green Version]
- Süntar, I.; Tumen, I.; Ustün, O.; Keleş, H.; Küpeli Akkol, E. Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of Pinus species by in vivo and in vitro experimental models. J. Ethnopharmacol. 2012, 139, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Ustun, O.; Senol, F.S.; Kurkcuoglu, M.; Orhan, I.E.; Kartal, M.; Baser, K.H.C. Investigation on chemical composition, anticholinesterase and antioxidant activities of extracts and essential oils of Turkish Pinus species and pycnogenol. Ind. Crops Prod. 2012, 38, 115–123. [Google Scholar] [CrossRef]
- Postu, P.A.; Sadiki, F.Z.; El Idrissi, M.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L. Pinus halepensis essential oil attenuates the toxic Alzheimer’s amyloid beta (1-42)-induced memory impairment and oxidative stress in the rat hippocampus. Biomed. Pharmacother. 2019, 112, 108673. [Google Scholar] [CrossRef]
- Postu, P.A.; Gorgan, D.L.; Cioanca, O.; Russ, M.; Mikkat, S.; Glocker, M.O.; Hritcu, L. Memory-enhancing effects of origanum majorana essential oil in an alzheimer’s amyloid beta1-42 rat model: A molecular and behavioral study. Antioxidants 2020, 9, 919. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Kodaira, N.; Tanaka, C.; Ishibashi, T.; Kudo, N.; Kawashima, Y.; Mitsumoto, A. Time of Administration of Acute or Chronic Doses of Imipramine Affects its Antidepressant Action in Rats. J. Circadian Rhythms 2018, 16, 5. [Google Scholar] [CrossRef]
- Wilson, M.A.; Burghardt, P.R.; Ford, K.A.; Wilkinson, M.B.; Primeaux, S.D. Anxiolytic effects of diazepam and ethanol in two behavioral models: Comparison of males and females. Pharmacol. Biochem. Behav. 2004, 78, 445–458. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. “Behavioural despair” in rats and mice: Strain differences and the effects of imipramine. Eur. J. Pharmacol. 1978, 51, 291–294. [Google Scholar] [CrossRef]
- Slattery, D.A.; Cryan, J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef]
- Mezadri, T.J.; Batista, G.M.; Portes, A.C.; Marino-Neto, J.; Lino-de-Oliveira, C. Repeated rat-forced swim test: Reducing the number of animals to evaluate gradual effects of antidepressants. J. Neurosci. Methods 2011, 195, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Winterbourn, C.; Hawkins, R.; Brian, M.; Carrell, R. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975, 85, 337. [Google Scholar] [PubMed]
- Fukuzawa, K.; Tokumura, A. Glutathione peroxidase activity in tissues of vitamin E-deficient mice. J. Nutr. Sci. Vitaminol. 1976, 22, 405–407. [Google Scholar] [CrossRef]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Salbitani, G.; Bottone, C.; Carfagna, S. Determination of Reduced and Total Glutathione Content in Extremophilic Microalga Galdieria phlegrea. Bio-Protocol 2017, 7, e2372. [Google Scholar] [CrossRef]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-related changes in oxidized proteins. J. Biol Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Postu, P.A.; Noumedem, J.A.K.; Cioanca, O.; Hancianu, M.; Mihasan, M.; Ciorpac, M.; Gorgan, D.L.; Petre, B.A.; Hritcu, L. Lactuca capensis reverses memory deficits in Aβ1-42-induced an animal model of Alzheimer’s disease. J. Cell. Mol. Med. 2018, 22, 111–122. [Google Scholar] [CrossRef]
- Magierski, R.; Sobow, T.; Schwertner, E.; Religa, D. Pharmacotherapy of Behavioral and Psychological Symptoms of Dementia: State of the Art and Future Progress. Front. Pharmacol. 2020, 11, 1168. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, R.; Taipale, H.; Tolppanen, A.M.; Tanskanen, A.; Tiihonen, J.; Hartikainen, S.; Koponen, M. Duration of new antidepressant use and factors associated with discontinuation among community-dwelling persons with Alzheimer’s disease. Eur. J. Clin. Pharmacol. 2019, 75, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Ettcheto, M.; Olloquequi, J.; Sánchez-López, E.; Busquets, O.; Cano, A.; Manzine, P.R.; Beas-Zarate, C.; Castro-Torres, R.D.; García, M.L.; Bulló, M.; et al. Benzodiazepines and Related Drugs as a Risk Factor in Alzheimer’s Disease Dementia. Front. Aging Neurosci. 2020, 11, 344. [Google Scholar] [CrossRef]
- Biedermann, S.V.; Biedermann, D.G.; Wenzlaff, F.; Kurjak, T.; Nouri, S.; Auer, M.K.; Wiedemann, K.; Briken, P.; Haaker, J.; Lonsdorf, T.B.; et al. An elevated plus-maze in mixed reality for studying human anxiety-related behavior. BMC Biol. 2017, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, N.E.; Barrow, J.C. Classics in Chemical Neuroscience: Diazepam (Valium). ACS Chem. Neurosci. 2014, 5, 253. [Google Scholar] [CrossRef]
- Arndt, D.L.; Peterson, C.J.; Cain, M.E. Differential Rearing Alters Forced Swim Test Behavior, Fluoxetine Efficacy, and Post-Test Weight Gain in Male Rats. PLoS ONE 2015, 10, e0131709. [Google Scholar] [CrossRef]
- Johnson, N.R.; Wang, A.C.-J.; Coughlan, C.; Sillau, S.; Lucero, E.; Viltz, L.; Markham, N.; Allen, C.; Dhanasekaran, A.R.; Chial, H.J.; et al. Imipramine and olanzapine block apoE4-catalyzed polymerization of Aβ and show evidence of improving Alzheimer’s disease cognition. Alzheimers Res. Ther. 2022, 14, 88. [Google Scholar] [CrossRef]
- Hwang, E.S.; Kim, H.B.; Lee, S.; Kim, M.J.; Kim, K.J.; Han, G.; Han, S.Y.; Lee, E.A.; Yoon, J.H.; Kim, D.O.; et al. Antidepressant-like effects of β-caryophyllene on restraint plus stress-induced depression. Behav. Brain Res. 2020, 380, 112439. [Google Scholar] [CrossRef]
- Aguilar-Ávila, D.S.; Flores-Soto, M.E.; Tapia-Vázquez, C.; Pastor-Zarandona, O.A.; López-Roa, R.I.; Viveros-Paredes, J.M. β-Caryophyllene, a Natural Sesquiterpene, Attenuates Neuropathic Pain and Depressive-Like Behavior in Experimental Diabetic Mice. J. Med. Food 2019, 22, 460–468. [Google Scholar] [CrossRef]
- Da Conceição Machado, K.; Paz, M.F.C.J.; de Oliveira Santos, J.V.; da Silva, F.C.C.; Tchekalarova, J.D.; Salehi, B.; Islam, M.T.; Setzer, W.N.; Sharifi-Rad, J.; de Castro e Sousa, J.M.; et al. Anxiety Therapeutic Interventions of β-Caryophyllene: A Laboratory-Based Study. Nat. Prod. Commun. 2020, 15, 1934578X2096222. [Google Scholar] [CrossRef]
- Szaszkiewicz, J.; Leigh, S.; Hamilton, T.J. Robust behavioural effects in response to acute, but not repeated, terpene administration in Zebrafish (Danio rerio). Sci. Rep. 2021, 11, 19214. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Shimada, A.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Matsumoto, Y.; Okamoto, M.; Ishihara, T. Attenuation Effects of Alpha-Pinene Inhalation on Mice with Dizocilpine-Induced Psychiatric-Like Behaviour. Evid. Based Complement. Altern. Med. 2019, 2019, 2745453. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Woo, J.; Pae, A.N.; Um, M.Y.; Cho, N.C.; Park, K.D.; Yoon, M.; Kim, J.; Lee, C.J.; Cho, S. α-Pinene, a Major Constituent of Pine Tree Oils, Enhances Non-Rapid Eye Movement Sleep in Mice through GABAA-benzodiazepine Receptors. Mol. Pharmacol. 2016, 90, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Walczyk-Mooradally, A.; Holborn, J.; Singh, K.; Tyler, M.; Patnaik, D.; Wesseling, H.; Brandon, N.J.; Steen, J.; Graether, S.P.; Haggarty, S.J.; et al. Phosphorylation-dependent control of Activity-regulated cytoskeleton-associated protein (Arc) protein by TNIK. J. Neurochem. 2021, 158, 1058–1073. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, M.E.; Gafford, G.M.; Jarome, T.J.; Helmstetter, F.J. Time-dependent expression of Arc and zif268 after acquisition of fear conditioning. Neural Plast. 2010, 2010, 139891. [Google Scholar] [CrossRef]
- Minatohara, K.; Akiyoshi, M.; Okuno, H. Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Front. Mol. Neurosci. 2016, 8, 78. [Google Scholar] [CrossRef]
- Jiang, Y.; Van Dongen, A.M.J. Selective increase of correlated activity in Arc-positive neurons after chemically induced long-term potentiation in cultured hippocampal neurons. eNeuro 2021, 8, ENEURO.0540-20.2021. [Google Scholar] [CrossRef]
- Abraham, W.C.; Jones, O.D.; Glanzman, D.L. Is plasticity of synapses the mechanism of long-term memory storage? Npj Sci. Learn. 2019, 4, 9. [Google Scholar] [CrossRef]
- Penrod, R.D.; Kumar, J.; Smith, L.N.; McCalley, D.; Nentwig, T.B.; Hughes, B.W.; Barry, G.M.; Glover, K.; Taniguchi, M.; Cowan, C.W. Activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) regulates anxiety- and novelty-related behaviors. Genes Brain Behav. 2019, 18, e12561. [Google Scholar] [CrossRef]
- Li, Y.; Pehrson, A.L.; Waller, J.A.; Dale, E.; Sanchez, C.; Gulinello, M. A critical evaluation of the activity-regulated cytoskeleton-associated protein (Arc/Arg3.1)’s putative role in regulating dendritic plasticity, cognitive processes, and mood in animal models of depression. Front. Neurosci. 2015, 9, 279. [Google Scholar] [CrossRef] [Green Version]
- Scarpa, J.R.; Jiang, P.; Gao, V.D.; Fitzpatrick, K.; Millstein, J.; Olker, C.; Gotter, A.; Winrow, C.J.; Renger, J.J.; Kasarskis, A.; et al. Cross-species systems analysis identifies gene networks differentially altered by sleep loss and depression. Sci. Adv. 2018, 4, eaat1294. [Google Scholar] [CrossRef] [PubMed]
- Gammie, S.C. Creation of a gene expression portrait of depression and its application for identifying potential treatments. Sci. Rep. 2021, 11, 3829. [Google Scholar] [CrossRef] [PubMed]
- Bohnsack, J.P.W.; Zhang, H.; Wandling, G.M.; He, D.; Kyzar, E.J.; Lasek, A.W.; Pandey, S.C. Targeted epigenomic editing ameliorates adult anxiety and excessive drinking after adolescent alcohol exposure. Sci. Adv. 2022, 8, 2748. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 575. [Google Scholar] [CrossRef]
- Hampel, H.; Caraci, F.; Cuello, A.C.; Caruso, G.; Nisticò, R.; Corbo, M.; Baldacci, F.; Toschi, N.; Garaci, F.; Chiesa, P.A.; et al. A Path Toward Precision Medicine for Neuroinflammatory Mechanisms in Alzheimer’s Disease. Front. Immunol. 2020, 11, 456. [Google Scholar] [CrossRef]
- Cadete Martini, A.; Gomez-Arboledas, A.; Forner, S.; Rodriguez-Ortiz, C.J.; McQuade, A.; Danhash, E.; Phan, J.; Javonillo, D.; Ha, J.-V.; Tram, M.; et al. Amyloid-beta impairs TOM1-mediated IL-1R1 signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 21198–21206. [Google Scholar] [CrossRef]
- Xie, L.; Lai, Y.; Lei, F.; Liu, S.; Liu, R.; Wang, T. Exploring the association between interleukin-1β and its interacting proteins in Alzheimer’s disease. Mol. Med. Rep. 2015, 11, 3219. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, Z.; Liu, S. β-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 Mice through CB2 receptor activation and the PPARγ pathway. Pharmacology 2014, 94, 1–12. [Google Scholar] [CrossRef]
- Khan-Mohammadi-Khorrami, M.K.; Asle-Rousta, M.; Rahnema, M.; Amini, R. Neuroprotective effect of alpha-pinene is mediated by suppression of the TNF-α/NF-κB pathway in Alzheimer’s disease rat model. J. Biochem. Mol. Toxicol. 2022, 36, e23006. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Sharma, N.; Khurana, N. Ameliorative effect of myrcene in mouse model of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 911, 174529. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef] [PubMed]
- Lloret, A.; Esteve, D.; Lloret, M.A.; Monllor, P.; López, B.; León, J.L.; Cervera-Ferri, A. Is Oxidative Stress the Link Between Cerebral Small Vessel Disease, Sleep Disruption, and Oligodendrocyte Dysfunction in the Onset of Alzheimer’s Disease? Front. Physiol. 2021, 12, 1330. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Gella, A.; Durany, N. Oxidative stress in Alzheimer disease. Cell Adhes. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef]
- Fakhri, S.; Pesce, M.; Patruno, A.; Moradi, S.Z.; Iranpanah, A.; Farzaei, M.H.; Sobarzo-Sánchez, E. Attenuation of Nrf2/Keap1/ARE in Alzheimer’s Disease by Plant Secondary Metabolites: A Mechanistic Review. Molecules 2020, 25, 4926. [Google Scholar] [CrossRef] [PubMed]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Major selected monoterpenes α-pinene and 1,8-cineole found in Salvia lavandulifolia (Spanish sage) essential oil as regulators of cellular redox balance. Pharm. Biol. 2015, 53, 921–929. [Google Scholar] [CrossRef]
- Xanthis, V.; Fitsiou, E.; Voulgaridou, G.P.; Bogadakis, A.; Chlichlia, K.; Galanis, A.; Pappa, A. Antioxidant and Cytoprotective Potential of the Essential Oil Pistacia lentiscus var. chia and Its Major Components Myrcene and α-Pinene. Antioxidants 2021, 10, 127. [Google Scholar] [CrossRef]
- Da Conceição Machado, K.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef]
- Ullah, H.; Di Minno, A.; Santarcangelo, C.; Khan, H.; Daglia, M. Improvement of Oxidative Stress and Mitochondrial Dysfunction by β-Caryophyllene: A Focus on the Nervous System. Antioxidants 2021, 10, 546. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R. A mechanistic review on immunomodulatory effects of selective type two cannabinoid receptor β-caryophyllene. Biofactors 2022, 48, 857–882. [Google Scholar] [CrossRef] [PubMed]
- Farmer, K.M.; Ghag, G.; Puangmalai, N.; Montalbano, M.; Bhatt, N.; Kayed, R. P53 aggregation, interactions with tau, and impaired DNA damage response in Alzheimer’s disease. Acta Neuropathol. Commun. 2020, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, D.L.; Piras, I.S.; Mastroeni, D.; Weisenberger, D.J.; Nolz, J.; Delvaux, E.; Serrano, G.E.; Beach, T.G.; Huentelman, M.J.; Coleman, P.D. Cell death and survival pathways in Alzheimer’s disease: An integrative hypothesis testing approach utilizing -omic data sets. Neurobiol. Aging 2020, 95, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Kang, R.; Fan, X.G.; Tang, D. Release and activity of histone in diseases. Cell Death Dis. 2014, 5, e1370. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, O.; Oztanir, M.N.; Cetin, A. Neuroprotective effects of β-myrcene following global cerebral ischemia/reperfusion-mediated oxidative and neuronal damage in a C57BL/J6 mouse. Neurochem. Res. 2014, 39, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Khoshnazar, M.; Parvardeh, S.; Bigdeli, M.R. Alpha-pinene exerts neuroprotective effects via anti-inflammatory and anti-apoptotic mechanisms in a rat model of focal cerebral ischemia-reperfusion. J. Stroke Cerebrovasc. Dis. 2020, 29, 104977. [Google Scholar] [CrossRef]
- Wang, G.; Ma, W.; Du, J. β-Caryophyllene (BCP) ameliorates MPP+ induced cytotoxicity. Biomed. Pharmacother. 2018, 103, 1086–1091. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postu, P.A.; Mihasan, M.; Gorgan, D.L.; Sadiki, F.Z.; El Idrissi, M.; Hritcu, L. Pinus halepensis Essential Oil Ameliorates Aβ1-42-Induced Brain Injury by Diminishing Anxiety, Oxidative Stress, and Neuroinflammation in Rats. Biomedicines 2022, 10, 2300. https://doi.org/10.3390/biomedicines10092300
Postu PA, Mihasan M, Gorgan DL, Sadiki FZ, El Idrissi M, Hritcu L. Pinus halepensis Essential Oil Ameliorates Aβ1-42-Induced Brain Injury by Diminishing Anxiety, Oxidative Stress, and Neuroinflammation in Rats. Biomedicines. 2022; 10(9):2300. https://doi.org/10.3390/biomedicines10092300
Chicago/Turabian StylePostu, Paula Alexandra, Marius Mihasan, Dragos Lucian Gorgan, Fatima Zahra Sadiki, Mostafa El Idrissi, and Lucian Hritcu. 2022. "Pinus halepensis Essential Oil Ameliorates Aβ1-42-Induced Brain Injury by Diminishing Anxiety, Oxidative Stress, and Neuroinflammation in Rats" Biomedicines 10, no. 9: 2300. https://doi.org/10.3390/biomedicines10092300