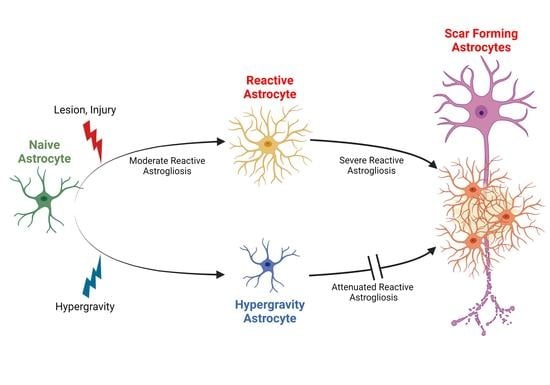
Hypergravity Attenuates Reactivity in Primary Murine Astrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Murine Cortical Astrocyte Isolation & Mouse Lines
2.2. Exposure to Hypergravity Conditions
2.2.1. MuSIC Incubator Centrifuge
2.2.2. The Hyperscope Live-Cell Imaging Platform on the Human Centrifuge
Live Spreading Rates
Wound-Healing Assay
LifeAct-GFP Dynamics
2.3. Staining & Image Analysis
2.3.1. Immunofluorescence Microscopy
2.3.2. Proliferation and Apoptosis Assays
2.3.3. Cytoskeletal Components and Reactivity Markers
2.3.4. Image Analysis
Morphological Parameters
Statistical Analysis
2.4. Biochemical Assessment of Protein Levels
3. Results
3.1. Increased Gravitational Load Induces Spreading Deficits in Primary Astrocytes
3.2. Acute and Persistently Impaired Migration Speed of Astrocytes Exposed to 2g Hypergravity
3.3. Morphology Alterations upon Hypergravity Exposure Revealed Decreased Cell Polarity
3.4. Actin Filament and Microtubule Dynamics Are Affected by 2g Hypergravity
3.5. Stable Expression Levels of Major Cytoskeletal Components
3.6. Proliferation and Apoptosis Rates Are Not Affected by Hypergravity
3.7. Hypergravity Attenuates Astrocyte Reactivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Pittà, M.; Brunel, N. Modulation of Synaptic Plasticity by Glutamatergic Gliotransmission: A Modeling Study. Neural Plast. 2016, 2016, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Abraham, W.C. Astrocytes and synaptic plasticity in health and disease. Exp. Brain Res. 2017, 235, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef]
- Adams, K.L.; Gallo, V. The diversity and disparity of the glial scar. Nat. Neurosci. 2017, 21, 9–15. [Google Scholar] [CrossRef]
- Burda, J.E.; Sofroniew, M.V. Reactive Gliosis and the Multicellular Response to CNS Damage and Disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef]
- Hur, E.-M.; Saijilafu; Zhou, F.-Q. Growing the growth cone: Remodeling the cytoskeleton to promote axon regeneration. Trends Neurosci. 2012, 35, 164–174. [Google Scholar] [CrossRef]
- Kamermans, A.; Planting, K.E.; Jalink, K.; Van Horssen, J.; De Vries, H.E. Reactive astrocytes in multiple sclerosis impair neuronal outgrowth through TRPM7-mediated chondroitin sulfate proteoglycan production. Glia 2018, 67, 68–77. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C.; et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study. Lancet Neurol. 2018, 18, 56–87. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Squair, J.W.; Gautier, M.; Sofroniew, M.V.; Courtine, G.; Anderson, M.A. Engineering spinal cord repair. Curr. Opin. Biotechnol. 2021, 72, 48–53. [Google Scholar] [CrossRef]
- Yang, T.; Dai, Y.; Chen, G.; Cui, S. Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The glia/neuron ratio: How it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia 2014, 62, 1377–1391. [Google Scholar] [CrossRef]
- Pekny, M.; Nilsson, M. Astrocyte activation and reactive gliosis. Glia 2005, 50, 427–434. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Etienne-Manneville, S.; Hall, A. Integrin-Mediated Activation of Cdc42 Controls Cell Polarity in Migrating Astrocytes through PKCζ. Cell 2001, 106, 489–498. [Google Scholar] [CrossRef]
- Okada, S.; Hara, M.; Kobayakawa, K.; Matsumoto, Y.; Nakashima, Y. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res. 2018, 126, 39–43. [Google Scholar] [CrossRef]
- Okada, S.; Nakamura, M.; Katoh, H.; Miyao, T.; Shimazaki, T.; Ishii, K.; Yamane, J.; Yoshimura, A.; Iwamoto, Y.; Toyama, Y.; et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 2006, 12, 829–834. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Reactive Astrocytes in Neural Repair and Protection. Neurosci. 2005, 11, 400–407. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Hlavac, N.; Guilhaume-Corrêa, F.; VandeVord, P.J. Mechano-stimulation initiated by extracellular adhesion and cationic conductance pathways influence astrocyte activation. Neurosci. Lett. 2020, 739, 135405. [Google Scholar] [CrossRef]
- Hlavac, N.; VandeVord, P.J. Astrocyte Mechano-Activation by High-Rate Overpressure Involves Alterations in Structural and Junctional Proteins. Front. Neurol. 2019, 10, 99. [Google Scholar] [CrossRef]
- Paemeleire, K.; Leybaert, L. Ionic changes accompanying astrocytic intercellular calcium waves triggered by mechanical cell damaging stimulation. Brain Res. 2000, 857, 235–245. [Google Scholar] [CrossRef]
- Genchi, G.G.; Rocca, A.; Marino, A.; Grillone, A.; Mattoli, V.; Ciofani, G. Hypergravity as a Tool for Cell Stimulation: Implications in Biomedicine. Front. Astron. Space Sci. 2016, 3, 26. [Google Scholar] [CrossRef]
- Häder, D.-P.; Braun, M.; Grimm, D.; Hemmersbach, R. Gravireceptors in eukaryotes-a comparison of case studies on the cellular level. Npj Microgravity 2017, 3, 13. [Google Scholar] [CrossRef]
- Horn, E. Fundamentals of Space Biology, 1st ed.; Springer: New York, NY, USA, 2006; Volume 18. [Google Scholar]
- Oman, M.C. Neurovestibular adaptation to spaceflight: Research progress. J. Vestib. Res. 2003, 12, 201–203. [Google Scholar] [CrossRef]
- Kanas, N.; Manzey, D. Basic issues of human adaptation to space flight. In Space Psychology and Psychiatry; Springer: Dordrecht, The Netherlands, 2008; Volume 22, pp. 15–48. [Google Scholar] [CrossRef]
- Koppelmans, V.; Bloomberg, J.J.; Mulavara, A.P.; Seidler, R.D. Brain structural plasticity with spaceflight. Npj Microgravity 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Van Ombergen, A.; Demertzi, A.; Tomilovskaya, E.; Jeurissen, B.; Sijbers, J.; Kozlovskaya, I.B.; Parizel, P.M.; Van De Heyning, P.H.; Sunaert, S.; Laureys, S.; et al. The effect of spaceflight and microgravity on the human brain. J. Neurol. 2017, 264, 18–22. [Google Scholar] [CrossRef]
- Frett, T.; Petrat, G.; van Loon, J.J.W.A.; Hemmersbach, R.; Anken, R. Hypergravity Facilities in the ESA Ground-Based Facility Program—Current Research Activities and Future Tasks. Microgravity Sci. Technol. 2015, 28, 205–214. [Google Scholar] [CrossRef]
- Burda, J.E.; O’Shea, T.M.; Ao, Y.; Suresh, K.B.; Wang, S.; Bernstein, A.M.; Chandra, A.; Deverasetty, S.; Kawaguchi, R.; Kim, J.H.; et al. Divergent transcriptional regulation of astrocyte reactivity across disorders. Nature 2022, 606, 557–564. [Google Scholar] [CrossRef]
- Schiweck, J.; Eickholt, B.J.; Murk, K. Important Shapeshifter: Mechanisms Allowing Astrocytes to Respond to the Changing Nervous System during Development, Injury and Disease. Front. Cell. Neurosci. 2018, 12, 261. [Google Scholar] [CrossRef]
- Deibler, M.; Spatz, J.P.; Kemkemer, R. Actin Fusion Proteins Alter the Dynamics of Mechanically Induced Cytoskeleton Rearrangement. PLoS ONE 2011, 6, e22941. [Google Scholar] [CrossRef]
- Riedl, J.; Crevenna, A.; Kessenbrock, K.; Yu, J.H.; Neukirchen, D.; Bista, M.; Bradke, F.; Jenne, D.; Holak, T.A.; Werb, Z.; et al. Lifeact: A versatile marker to visualize F-actin. Nat. Methods 2008, 5, 605–607. [Google Scholar] [CrossRef]
- Riedl, J.; Flynn, K.C.; Raducanu, A.; Gärtner, F.; Beck, G.; Bösl, M.; Bradke, F.; Massberg, S.; Aszodi, A.; Sixt, M.; et al. Lifeact mice for studying F-actin dynamics. Nat. Methods 2010, 7, 168–169. [Google Scholar] [CrossRef]
- Corydon, T.J.; Kopp, S.; Wehland, M.; Braun, M.; Schütte, A.; Mayer, T.; Hülsing, T.; Oltmann, H.; Schmitz, B.; Hemmersbach, R.; et al. Alterations of the cytoskeleton in human cells in space proved by life-cell imaging. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Middeldorp, J.; Hol, E.M. GFAP in health and disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef]
- Bonfiglio, T.; Biggi, F.; Bassi, A.M.; Ferrando, S.; Gallus, L.; Loiacono, F.; Ravera, S.; Rottigni, M.; Scarfì, S.; Strollo, F.; et al. Simulated microgravity induces nuclear translocation of Bax and BCL-2 in glial cultured C6 cells. Heliyon 2019, 5, e01798. [Google Scholar] [CrossRef] [PubMed]
- Cubano, L.A.; Lewis, M.L. Fas/APO-1 protein is increased in spaceflown lymphocytes (Jurkat). Exp. Gerontol. 2000, 35, 389–400. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, E. Transcriptional Analysis of Normal Human Fibroblast Responses to Microgravity Stress. Genom. Proteom. Bioinform. 2008, 6, 29–41. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, H.; Wu, L.; Cao, L.; Yang, Q.; Dong, H.; Wang, Z.; Ma, J.; Li, Z. The influence of simulated microgravity on proliferation and apoptosis in U251 glioma cells. In Vitro Cell. Dev. Biol.-Animal 2017, 53, 744–751. [Google Scholar] [CrossRef]
- Schutte, B.; Nuydens, R.; Geerts, H.; Ramaekers, F. Annexin V binding assay as a tool to measure apoptosis in differentiated neuronal cells. J. Neurosci. Methods 1998, 86, 63–69. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Escartin, C.; Guillemaud, O.; Carrillo-de Sauvage, M.-A. Questions and (some) answers on reactive astrocytes. Glia 2019, 67, 2221–2247. [Google Scholar] [CrossRef]
- Chen, M.; Geoffroy, C.G.; Meves, J.M.; Narang, A.; Li, Y.; Nguyen, M.T.; Khai, V.S.; Kong, X.; Steinke, C.L.; Carolino, K.I.; et al. Leucine Zipper-Bearing Kinase Is a Critical Regulator of Astrocyte Reactivity in the Adult Mammalian CNS. Cell Rep. 2018, 22, 3587–3597. [Google Scholar] [CrossRef]
- Clemente, C.D.; Windle, W.F. Regeneration of severed nerve fibers in the spinal cord of the adult cat. J. Comp. Neurol. 1954, 101, 691–731. [Google Scholar] [CrossRef]
- Sugar, O.; Gerard, R.W. Spinal cord regeneration in the rat. J. Neurophysiol. 1940, 3, 1–19. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Dissecting spinal cord regeneration. Nature 2018, 557, 343–350. [Google Scholar] [CrossRef]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef]
- Anderson, M.A.; O’Shea, T.M.; Burda, J.E.; Ao, Y.; Barlatey, S.L.; Bernstein, A.M.; Kim, J.H.; James, N.D.; Rogers, A.; Kato, B.; et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 2018, 561, 396–400. [Google Scholar] [CrossRef]
- Gu, Y.; Cheng, X.; Huang, X.; Yuan, Y.; Qin, S.; Tan, Z.; Wang, D.; Hu, X.; He, C.; Su, Z. Conditional ablation of reactive astrocytes to dissect their roles in spinal cord injury and repair. Brain Behav. Immun. 2019, 80, 394–405. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Courtine, G.; Sofroniew, M.V. Spinal cord repair: Advances in biology and technology. Nat. Med. 2019, 25, 898–908. [Google Scholar] [CrossRef]
- Griffin, J.M.; Bradke, F. Therapeutic repair for spinal cord injury: Combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020, 12, e11505. [Google Scholar] [CrossRef]
- Tedeschi, A.; Bradke, F. Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr. Opin. Neurobiol. 2017, 42, 118–127. [Google Scholar] [CrossRef]
- Bradke, F.; Dotti, C.G. The Role of Local Actin Instability in Axon Formation. Science 1999, 283, 1931–1934. [Google Scholar] [CrossRef]
- Coles, C.H.; Bradke, F. Coordinating Neuronal Actin–Microtubule Dynamics. Curr. Biol. 2015, 25, R677–R691. [Google Scholar] [CrossRef]
- Smith, D.S.; Skene, J.H. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J. Neurosci. 1997, 17, 646–658. [Google Scholar] [CrossRef]
- Hellal, F.; Hurtado, A.; Ruschel, J.; Flynn, K.C.; Laskowski, C.J.; Umlauf, M.; Kapitein, L.C.; Strikis, D.; Lemmon, V.; Bixby, J.; et al. Microtubule Stabilization Reduces Scarring and Causes Axon Regeneration After Spinal Cord Injury. Science 2011, 331, 928–931. [Google Scholar] [CrossRef]
- Ruschel, J.; Hellal, F.; Flynn, K.C.; Dupraz, S.; Elliott, D.A.; Tedeschi, A.; Bates, M.; Sliwinski, C.; Brook, G.; Dobrindt, K.; et al. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 2015, 348, 347–352. [Google Scholar] [CrossRef]
- Sengottuvel, V.; Leibinger, M.; Pfreimer, M.; Andreadaki, A.; Fischer, D. Taxol Facilitates Axon Regeneration in the Mature CNS. J. Neurosci. 2011, 31, 2688–2699. [Google Scholar] [CrossRef]
- Witte, H.; Neukirchen, D.; Bradke, F. Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 2008, 180, 619–632. [Google Scholar] [CrossRef]
- Shinde, V.; Brungs, S.; Henry, M.; Wegener, L.; Nemade, H.; Rotshteyn, A.; Acharya, A.; Baumstark-Khan, C.; Hellweg, C.E.; Hescheler, J.; et al. Simulated Microgravity Modulates Differentiation Processes of Embryonic Stem Cells. Cell. Physiol. Biochem. 2016, 38, 1483–1499. [Google Scholar] [CrossRef]
- Vassy, J.; Portet, S.; Beil, M.; Millot, G.; Fauvel-Lafève, F.; Karniguian, A.; Gasset, G.; Irinopoulou, T.; Calvo, F.; Rigaut, J.P.; et al. The effect of weightlessness on cytoskeleton architecture and proliferation of human breast cancer cell line MCF-7. FASEB J. 2001, 15, 1104–1106. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lichterfeld, Y.; Kalinski, L.; Schunk, S.; Schmakeit, T.; Feles, S.; Frett, T.; Herrmann, H.; Hemmersbach, R.; Liemersdorf, C. Hypergravity Attenuates Reactivity in Primary Murine Astrocytes. Biomedicines 2022, 10, 1966. https://doi.org/10.3390/biomedicines10081966
Lichterfeld Y, Kalinski L, Schunk S, Schmakeit T, Feles S, Frett T, Herrmann H, Hemmersbach R, Liemersdorf C. Hypergravity Attenuates Reactivity in Primary Murine Astrocytes. Biomedicines. 2022; 10(8):1966. https://doi.org/10.3390/biomedicines10081966
Chicago/Turabian StyleLichterfeld, Yannick, Laura Kalinski, Sarah Schunk, Theresa Schmakeit, Sebastian Feles, Timo Frett, Harald Herrmann, Ruth Hemmersbach, and Christian Liemersdorf. 2022. "Hypergravity Attenuates Reactivity in Primary Murine Astrocytes" Biomedicines 10, no. 8: 1966. https://doi.org/10.3390/biomedicines10081966