Pain and Opioid-Induced Gut Microbial Dysbiosis
Abstract
:1. Introduction
2. The Pain Pathway and Hyperalgesia
2.1. Opioid-Induced Hyperalgesia
2.2. Experimental Evidence
2.3. Antinociceptive Tolerance to Opioids
3. The Gut-Brain Axis, Withdrawal and Diseases
3.1. Addiction
3.2. Opioid Withdrawal
3.3. Inflammatory Bowel Disease
3.4. Parkinson’s Disease
3.5. Gut Function and Mental Health
4. Gut Homeostasis and the Epithelial Barrier
4.1. Impairment of Gut Epithelial Integrity
4.2. Intestinal Function Modulation
4.3. Host Immune System Modulation
4.4. Epithelial Barrier Function
4.5. Morphine and the Gut Microbiome and Metabolome
5. Inflammation and Infection
5.1. Chronic Opioid Use and Immunosuppression
5.2. Human Immunodeficiency Virus (HIV)
5.3. Hepatitis C Virus (HCV)
5.4. Herpes Simplex Virus
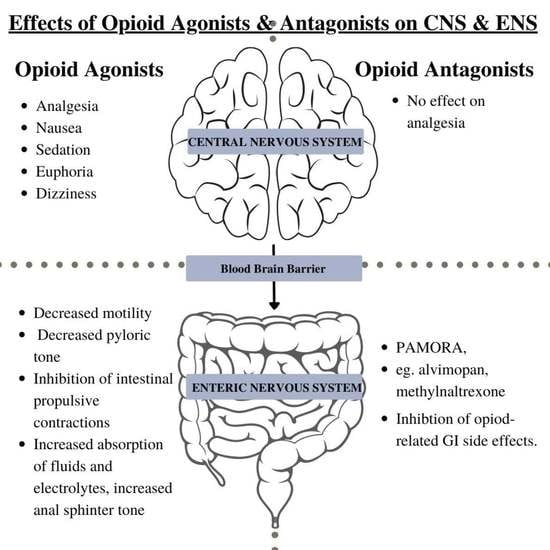
6. Gastrointestinal Motility and Pathophysiology of the Gut by Opioids
6.1. Opioid-Induced Bowel Dysfunction (OIBD)
6.2. Opioid-Induced Constipation (OIC)
7. Therapeutic Options and Treatment Perspectives
7.1. Faecal Microbiota Transplantation (FMT)
7.2. Antibiotic Treatment
7.3. Probiotic & Prebiotic Therapy
| Probiotics | Function |
|---|---|
| Bifidobacteria and Lactobacillaeae | Tolerant to morphine, and probiotics containing these bacterial communities can prevent the development of analgesic tolerance in the morphine-treated rats [66]. |
| B. longum and L. rhamnosus | Maintain a healthy intestinal barrier [68] bacterial translocation, and neuroinflammation by manipulating the gut microbiome [69]. |
| Lactobacillus genus | In mice, it was shown to induce opioid and cannabinoid CB2 receptor expression and mediate analgesic activities in intestinal epithelial cells [70]. |
7.4. Myosin Light Chain Kinase (MLCK) Inhibitor ML-7
7.5. Opioid-Induced Constipation (OIC)
| Microbial | Effect on the Gut |
|---|---|
| Mycobacterium | Major pathogen of IBD [26] |
| Escherichia coli | Major pathogen of IBD [26] |
| Clostridium difficile | Major pathogens of IBD [26] |
| Lactobacillaceae | Association with Parkinson disease [27] |
| Enterococcaceae | Association with Parkinson disease [27] |
| Alistipes | Increases prevalence in depression [78] |
| Faecalibacterium prausnitzii | Has anti-inflammatory properties that promote gut health. Decrease in this bacteria can have consequences on the epithelial integrity of the gut [46]. |
| Human Immunodeficiency Virus (HIV) | HIV is a virus that attacks its own immune system. Opioids have been associated with aiding the development of HIV in the CNS and worsening the neurodegenerative diseases that are caused by chronic HIV [53]. |
| Hepatitis C virus (HCV) | Opioid abusers may be regularly involved in needle use, sharing and hazardous disposal, and unsafe sex. These actions can easily expose them to HIV and blood-borne HCV [52]. |
| Herpes Simplex Virus | Herpes simplex virus (HSV) covers a range of infectious agents which cause oral and genital lesions, encephalitis, infections in neonates and malignant growths. Opioids delay the HSV clearance, alter the virus itself and reactivate latent HSV [52]. |
| Bifidobacterium genus | Probiotics that normally live in the gut [22]. |
8. Future Directions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine |
| Ach | Acetylcholine |
| AIDS | Acquired Immune Deficiency Syndrome |
| BDNF | Brain Derived Neurotrophic Factor |
| CNS | Central Nervous System |
| DOR | δ-opioid receptors |
| ENS | Enteric Nervous System |
| FMT | Faecal Microbiota Transplantation |
| GI | Gastrointestinal |
| HCV | Hepatitis C Virus |
| HIV | Human Immunodeficiency Virus |
| HPA | Hypothalamus Pituitary Axis |
| HSV | Herpes Simplex Virus |
| IBD | Irritable Bowel Disease |
| MLCK | Myosin Light Chain Kinase |
| MOR | μ-opioid receptors |
| NMDA | N-methyl-D-aspartate |
| OIBD | Opioid-Induced Bowel Dysfunction |
| OIC | Opioid Induced Constipation |
| OID | Opioid Induced Dysbiosis |
| OIH | Opioid-Induced hyperalgesia |
| OUD | Opioid Use Disorder |
| PAMORA | Peripherally Acting μ Opioid Receptor Antagonists |
| PNS | Peripheral Nervous System |
| QSP | Quantitative Systems Pharmacology |
| RVM | Rostral Ventromedial Medulla |
| TLR | Toll-Like Receptor |
| VIP | Vasoactive Intestinal Peptide |
References
- Foley, K.M. Opioids. Neurol. Clin. 1993, 11, 503–522. [Google Scholar] [CrossRef]
- Vadivelu, N.; Whitney, C.J.; Sinatra, R.S. Painpathways and acute pain processing. Acute Pain Manag. 2009, 27, 3–20. [Google Scholar]
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef]
- Zollner, C.; Stein, C. Opioids. Handb. Exp. Pharmacol. 2017, 177, 31–63. [Google Scholar]
- Wang, F.; Roy, S. Gut Homeostasis, Microbial Dysbiosis, and Opioids. Toxicol. Pathol. 2016, 45, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.; Shi, J.; Mehwish, H.M.; Zhu, J.; Li, Q.; Shao, D.; Huang, Q.; Yang, H. Interaction between diet composition and gut microbiota and it’s impact on gastrointestinal tract health. Food Sci. Hum. Wellness 2017, 6, 121–130. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Annols Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Cross, S.A. Pathophysiology of Pain. Mayo Clin. Proc. 1994, 69, 375–383. [Google Scholar] [CrossRef]
- Kanjhan, R. Opioids and Pain. Clin. Exp. Pharamcol. Physiol. 1995, 22, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Marion, L.; Silverman, S.M.; Hansen, H.; Patel, V.; Manchikanti, L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011, 14, 145–161. [Google Scholar]
- Ossipov, M.H.; Lai, J.; King, T.; Venderah, T.W.; Malan, T.P.J.; Hruby, V.J.; Porreca, F. Antinociceptive and nociceptive actions of opioids. J. Neurobiol. 2004, 61, 126–148. [Google Scholar] [CrossRef] [PubMed]
- Marshall, T.M.; Herman, D.S.; Largent-Milnes, T.M.; Badghisi, H.; Zuber, K.; Holt, S.C.; Lai, J.; Porreca, F.; Vanderah, T.W. Activation of descending pain- facilitatory pathways from the rostral ventromedial medulla by cholecystokinin elicits release of prostaglandin-e2 in the spinal cord. Pain 2012, 153, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, I.D.; Harasawa, I. Chronic morphine exposure increases the proportion of on-cells in the rostral ventromedial medulla in rats. Life Sci. 2007, 80, 1915–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angst, M.S.; Clark, J.D. Opioid-induced hyperalgesia: A qualitative and systematic review. Anesthesiology 2006, 104, 570–587. [Google Scholar] [CrossRef]
- Arner, S.; Rawal, N.; Gustafsson, L.L. Clinical experience of long–term treatment with epidural and intrathecal opioids–a nationwide survey. Acta Anaesthesiol. Scand. 1988, 32, 253–259. [Google Scholar] [CrossRef]
- Sjøgren, P.; Thunedborg, L.P.; Christrup, L.; Hansen, S.H.; Franks, J. Is development of hyperalgesia, allodynia and myoclonus related to morphine metabolism during long-term administration?: Six case histories. Acta Anaesthesiol. Scand. 2008, 42, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.M. Opioid induced hyperalgesia: Clinical implications for the pain practitioner. Pain Physician 2009, 12, 679–684. [Google Scholar] [CrossRef]
- Kang, M.; Mischel, R.A.; Bhave, S.; Komla, E.; Cho, A.; Huang, C.; Akbarali, H.I. The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci. Rep. 2017, 7, 42658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bastard, Q.; Al-Ghalith, G.A.; Gregoire, M.; Chapelet, G.; Javaudin, F.; Dailly, E.; Batard, E.; Knights, D.; Montassier, E. Sytematic review: Human gut dysbiosis induced by non-antibiotic prescription medications. Aliment. Pharmacol. Ther. 2017, 47, 332–345. [Google Scholar] [CrossRef] [Green Version]
- Rogers, G.; Keating, D.; Young, R.; Wong, M.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [Green Version]
- Russell, J.T.; Zhou, Y.; Weinstock, G.M.; Bubier, J.A. The Gut Microbiome and Substance Use Disorders. Front. Neurosci. 2021, 15, 725500. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.J.; Malahias, E.; Park, J.; Srivastava, A.; Reyes, B.A.S.; Gorky, J.; Vadigepalli, R.; van Bockstaele, E.J.; Schwaber, J.S. Single-Cell Glia and Neuron Gene Expression in the Central Amygdala in Opioid Withdrawal Suggests Inflammation with Correlated Gut Dysbiosis. Front. Neurosci. 2019, 13, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yang, J.; Yang, C.; Chen, T.; Wang, Z.; Li, J.; Qin, F.; Deng, Q.; Zhang, X. Sensitivity to Morphine Reward Associates With Gut Dysbiosis in Rats With Morphine-Induced Conditioned Place Preference. Front. Psychiatry 2020, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Truant, A.L.; Meissler, J.J.; Gaughan, J.P.; Adler, M.W.; Eisenstein, T.K. Morphine Withdrawal Lowers Host Defense to Enteric Bacteria: Spontaneous Sepsis and Increased Sensitivity to Oral Salmonella Enterica Serovar Thphimurium Infection. Infect. Immun. 2006, 74, 5221–5226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.; Huecker, M.R. Opioid Withdrawal; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- De Gruttola, A.K.; Low, D.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [Green Version]
- Rueda-Ruzafa, L.; Cruz, F.; Cardona, D.; Hone, A.J.; Molina-Torres, G.; Sanchez-Labrace, N.; Roman, P. Opioid system influences gut-brain axis: Dysbiosis and related alterations. Pharamcaol. Res. 2020, 159, 104928. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Engen, P.; Bonvegna, S.; Cilia, R. The gut microbiome in Parkinson’s disease: A culprit or a bystander? Prog. Brain Res. 2020, 252, 357–450. [Google Scholar]
- Yellowlees, D. Gut Inflammation Linker to the Development of Parkinson’s Disease. 14 January 2020. Available online: https://www.psychiatryadvisor.com/home/topics/neurocognitive-disorders/gut-inflammation-linked-to-the-development-of-parkinson-disease/#:~:text=Gut%20Inflammation%20Linked%20to%20the%20Development%20of%20Parkinson%20Disease,-Yellowlees%20Douglas%2C%20PhD& (accessed on 14 June 2022).
- Safadi, J.M.; Quinton, A.M.G.; Lennox, B.R.; Burnet, P.W.J.; Minichino, A. Gut dysbiosis in severe mental illness and chronic fatigue: A novel trans-diagnostic construct? A systematic review and meta-analysis. Mol. Psychiatry 2021, 27, 141–153. [Google Scholar] [CrossRef]
- Mayer, E.; Savidge, T.; Sulman, R. Brain-Gut Microbiome Interactions and Functional Bowel Disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef] [Green Version]
- Lutz, P.; Kieffer, B. Opioid receptors: Distinct roles in mood disorders. Trends Neurosci. 2013, 36, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the gut microbiota regulate host serotnin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Parker, B.; Wearsch, A.; Veloo, A.; Rodriguez-Palacios, A. The Genus Alistipes Gut Bacteria With Emerging Implications to Inflammation, Cancer and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Best, J.; Nijhout, H.F.; Reed, M. Serotonin synthesis, release and re-uptake in terminals: A mathematical model. Theor. Biol. Med. Model. 2010, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.-M.; Lee, K.-E.; Kim, D.-H. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients 2019, 11, 819. [Google Scholar] [CrossRef] [Green Version]
- American Chemical Society. Negative Side Effects of Opioids Could Be Coming from Users’ Own Immune Systems. 17 August 2020. Available online: https://www.acs.org/content/acs/en/pressroom/newsreleases/2020/august/negative-side-effects-of-opioids-could-be-coming-from-users-own-immune-systems-video.html (accessed on 14 June 2022).
- Rahman, M.T.; Ghosh, C.; Hossain, M.; Linfield, D.; Rezaee, F.; Janigro, D.; Marchi, N.; Boxel-Dezaire, A.H.H. IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem. Biophys. Res. Commun. 2018, 9, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017, 20, 1722–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brites, D.; Fernandes, A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front. Cell. Neurosci. 2015, 9, 476. [Google Scholar] [CrossRef] [Green Version]
- Juruena, F.; Bocharova, M.; Agustini, B.; Young, A. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. J. Affect. Disord. 2018, 233, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Sobczak, M.; Salaga, M.; Storr, M.A.; Fichna, J. Physiology, signalling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: Current concepts and future perspectives. J. Gastroenterol. 2013, 49, 24–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iob, E.; Kirschbaum, C.; Steptoe, A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: The role of cognitive-affective and somatic symptoms. Mol. Psychiatry 2020, 25, 1130–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoruts, A.; Sadowsky, M. Understanding the mechanisms of faecal microbiota transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Muchhala, K.H.; Jacob, J.C.; Kang, M.; Dewey, W.L.; Akbarali, H.I. The Guts of the Opioid Crisis. Physiology 2021, 36, 315–323. [Google Scholar] [CrossRef]
- Meng, J.; Sindberg, G.M.; Roy, S. Disruption of gut homeostasis by opioids acceletates HIV disease progression. Front. Microbiol. 2015, 6, 643. [Google Scholar] [CrossRef] [Green Version]
- Schulzke, J.D.; Ploeger, S.; Amasheh, M.; Fromm, A.; Zeissig, S.; Troeger, H.; Richter, J. Epithelial tight junctions in intestinal inflammation. Ann. N. Y. Acad. Sci. 2009, 1165, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Yu, H.; Ma, J.; Wang, J.; Banerjee, S.; Charboneua, R.; Barke, R.A.; Roy, S. Morphine Induces Bacterial Translocation in Mice by Compromising Intestinal Barrier Function in a TLR-Dependent Manner. PLoS ONE 2013, 8, e54040. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Meng, J.; Zhang, L.; Johnson, T.; Chen, C.; Roy, S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci. Rep. 2018, 8, 3596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, A.L.; Curtis, J. Opioid use in Rheumatoid Arthritis: Trends, efficacy, safety and best practices. Curr. Opin. Rheumatol. 2019, 31, 264–270. [Google Scholar] [CrossRef]
- Tahamatan, A.; Tavakoli-Yaraki, M.; Mokhtari-Azad, T.; Teymoori-Rad, M.; Bont, L.; Shokri, F.; Salimi, V. Opiois and Viral Infections: A Double-Edged Sword. Front. Microbiol. 2016, 7, 970. [Google Scholar]
- Lui, B.; Lui, X.; Tang, S.J. Interactions of Opioids and HIV Infection in the Pathogenesis of Chronic Pain. Front. Microbiol. 2016, 7, 103. [Google Scholar]
- Akbarali, H.I.; Dewey, W.L. Gastrointestinal motility, dysbiosis and opioid-induced tolerance: Is there a link? Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 323–324. [Google Scholar] [CrossRef]
- Nelson, A.D.; Camilleri, M. Chronic opioid constipation in patients with nonmalignant pain: Challenges and opportunities. Ther. Adv. Gastroenterol. 2015, 8, 206–220. [Google Scholar]
- Galligan, J.J.; Sternini, C. Insights into the Role of Opioid Receptors in the GI Tract: Experimental Evidence and Therapeutic Relevance. Handb. Exp. Pharmacol. 2017, 239, 363–378. [Google Scholar]
- Sharp, B.M.; Roy, S.; Bidlack, J.M. Evidence for opioid receptors on cells involved in host defense and the immune system. J. Neuroimmunol. 1998, 83, 45–56. [Google Scholar] [CrossRef]
- Imam, M.Z.; Kuo, A.; Ghassabian, S.; Smith, M.T. Progress in understanding mechanisms of opioid-induced gastrointestinal adverse effects and respiratory depression. Neuropharmacology 2018, 131, 238–255. [Google Scholar] [CrossRef]
- Akbarali, H.I.; Dewey, W.L. The gut-brain interaction in opioid tolerance. Curr. Opin. Pharmacol. 2017, 37, 126–130. [Google Scholar]
- Nelson, A.D.; Camilleri, M. Opioid-induced constipation: Advances and clinical guidance. Ther. Adv. Chronic Dis. 2016, 7, 121–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller-Lissner, S.; Bassotti, G.; Coffin, B.; Drewes, A.M.; Breivik, H.; Eisenberg, E.; Emmanuel, A.; Laroche, F.; Meissner, W.; Morloin, B. Opioid-Induced Constipation and Bowel Dysfunction: A Clinical Guideline. Pain Med. 2016, 18, 1837–1863. [Google Scholar]
- Collett, B.J. Opioid tolerance: The clinical perspective. Br. J. Anaesth. 1998, 81, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hales, T.G. Arresting the development of morphine tolerance and dependence. Br. J. Anaesthesis 2011, 107, 653–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomaz, A.C.; Iyer, V.; Woodward, T.J.; Hohmann, A.G. Fecal microbiota transplantation and antibiotic treatment attenuate naloxone-precipitated opioid withdrawal in morphine-dependent mice. Exp. Neurol. 2021, 343, 113787. [Google Scholar] [CrossRef]
- Zhang, J.; Deji, C.; Fan, J.; Chang, L.; Miao, X.; Xiao, Y.; Zhu, Y.; Li, S. Differential alteration in gut microbiome profiles during acquisition, extinction and reinstatement of morphine-induced CCP. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110058. [Google Scholar] [CrossRef]
- Williams, N. Probiotics. Am. J. Health-Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Narbad, A.; Chen, W. A mixture of Lactobacillus species isolated from traditional fermented foods promote recovery from antibiotic-induced intestinal disruption in mice. J. Appl. Microbiol. 2018, 124, 842–854. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocriniology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Rousseaux, C.; Thuru, X.; Gelot, A.; Barnich, N.; Neut, C.; Dubuquoy, L.; Dubuquoy, C.; Merour, E.; Geboes, K.; Chamaillard, M.; et al. Lactobacillus acidophilus modulates intestinal pain and induces opiod and cannabinoid receptors. Nat. Med. 2007, 13, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Manning, T.; Gibson, G. Prebiotics. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 287–298. [Google Scholar] [CrossRef]
- Abot, A.; Wemelle, E.; Laurens, C.; Paquot, A.; Pomie, N.; Carper, D.; Bessac, A.; Orea, X.M.; Fremez, C.; Fontanie, M.; et al. Identification of new enterosynes using prebiotics: Roles of bioactive lipids and mu-opiod receptor signalling in humans and mice. Gut 2020, 70, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Thapa, N.; Kappus, M.; Hurt, R.; Diamond, S. Implications of the Opioid Epidemic for the Clinical Gastroenterology Practice. Curr. Gastroenterol. Rep. 2019, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Bragg, D.; El-Sharkawy, A.M.; Psaltis, E.; Maxwell-Armstrong, C.A.; Lobo, D.N. Postoperative ileus: Recent developments in pathophysiology and management. Clin. Nutr. 2015, 34, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Mesia, R.; Virizuela Echaburu, J.A.; Gomez, J.; Sauri, T.; Serrano, G.; Pujol, E. Opioid-Induced Constipation in Oncological Patients: New Strategies of Management. Curr. Treat. Options Oncol. 2019, 20, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crouse, B.; Zhang, L.; Robinson, C.; Ban, Y.; Vigliaturo, J.R.; Roy, S.; Pravetoni, M. Housing Conditions and microbial environment do not affect the efficacy of vaccines for treatment of opioid use disorders in mice and rats. Hum. Vaccines Immunother. 2021, 7, 4383–4392. [Google Scholar] [CrossRef]
- Lim, S.Y. Opioid Effects on the Central Nervous System and the Peripheral Immune System: Implications for Opioid Tolerance. Curr. Pharamcol. Rep. 2021, 7, 81–95. [Google Scholar] [CrossRef]
- Banerjee, S.; Sindberg, G.; Wang, F.; Meng, J.; Sharma, U.; Zhang, L.; Dauer, P.; Chen, C.; Dalluge, J.; Johnson, T.; et al. Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol. 2016, 9, 1418–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, K.E.; Sugiarto, E.; Taylor, A.M.; Pradhan, A.A.; Al-Hasani, R. Pain, Motivation, migraine, and the microbiome: New frontiers for opioid systems and disease. Mol. Pharmacol. 2020, 98, 433–444. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mischel, R.A.; Muchhala, K.H.; Dewey, W.L.; Akbarali, H.I. The “Culture” of Pain Control: A Review of Opioid-Induced Dysbiosis (OID) in Antinociceptive Tolerance. J. Pain 2020, 21, 751–762. [Google Scholar] [CrossRef]
- Stein, C. Opioid Receptors on Peripheral Sensory Neurons. In Madame Curie Bioscience Database, 2000–2013; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Mercandante, S.; Arcuri, E.; Santoni, A. Opioid-Induced Tolerance and Hyperalgesia. CNS Drugs 2019, 33, 943–955. [Google Scholar] [CrossRef]
- Holmes, A.; Finger, C.; Morales-Scheihing, D.; Lee, J.; McCullough, L.D. Gut dysbioisis and age-related neurological diseases; an innovative approach for therapeutic interventions. Transl. Res. 2020, 226, 39–56. [Google Scholar] [CrossRef]
- Jalodia, R.; Abu, Y.F.; Oppenheimer, M.R.; Herlihy, B.; Meng, J.; Chupikova, I.; Tao, J.; Ghosh, N.; Dutta, R.K.; Kolli, U.; et al. Opioid use, Gut Dysbiosis, Inflammation, and the Nervous System. Neuroimmune Pharmacol. 2022, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, K.R.; Watt, J.; Wu, C.M.J.; Akinrinoye, A.; Amjad, S.; Colvin, L.; Cowe, R.; Duncan, S.H.; Russell, W.R.; Forget, P. Pain and Opioid-Induced Gut Microbial Dysbiosis. Biomedicines 2022, 10, 1815. https://doi.org/10.3390/biomedicines10081815
Thomas KR, Watt J, Wu CMJ, Akinrinoye A, Amjad S, Colvin L, Cowe R, Duncan SH, Russell WR, Forget P. Pain and Opioid-Induced Gut Microbial Dysbiosis. Biomedicines. 2022; 10(8):1815. https://doi.org/10.3390/biomedicines10081815
Chicago/Turabian StyleThomas, Karen R., Jacob Watt, Chuen Mong J. Wu, Adejoke Akinrinoye, Sairah Amjad, Lucy Colvin, Rachel Cowe, Sylvia H. Duncan, Wendy R. Russell, and Patrice Forget. 2022. "Pain and Opioid-Induced Gut Microbial Dysbiosis" Biomedicines 10, no. 8: 1815. https://doi.org/10.3390/biomedicines10081815