Higher Responsiveness for Women, High Transaminase Levels, and Fat Percentage to Pemafibrate Treatment for NAFLD
Abstract
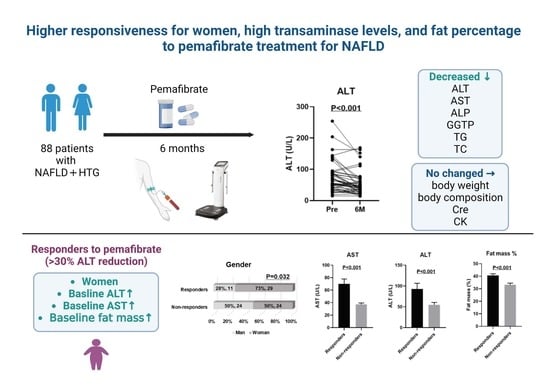
:1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Examinations
2.2. Body Composition Analysis
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics of HTG-NAFLD Patients Treated with PEM
3.2. Six-Month Treatment with PEM Significantly Improved Liver Function and Lipid Profiles
3.3. Comparison between Responders and Non-Responders to PEM Treatment in HTG-NAFLD Patients
3.4. ROC Curve Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, T.; Singh, S.; Tanaka, N.; Umemura, T. Role of G Protein-Coupled Receptors in Hepatic Stellate Cells and Approaches to Anti-Fibrotic Treatment of Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2021, 12, 773432. [Google Scholar] [CrossRef]
- Aomura, D.; Harada, M.; Yamada, Y.; Nakajima, T.; Hashimoto, K.; Tanaka, N.; Kamijo, Y. Pemafibrate Protects against Fatty Acid-Induced Nephropathy by Maintaining Renal Fatty Acid Metabolism. Metabolites 2021, 11, 372. [Google Scholar] [CrossRef]
- Zhang, Z.; Diao, P.; Zhang, X.; Nakajima, T.; Kimura, T.; Tanaka, N. Clinically Relevant Dose of Pemafibrate, a Novel Selective Peroxisome Proliferator-Activated Receptor α Modulator (SPPARMα), Lowers Serum Triglyceride Levels by Targeting Hepatic PPARα in Mice. Biomedicines 2022, 10, 1667. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as metabolic regulators in the liver: Lessons from liver-specific PPAR-null mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Aoyama, T.; Kimura, S.; Gonzalez, F.J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol. Ther. 2017, 179, 142–157. [Google Scholar] [CrossRef]
- Honda, Y.; Kessoku, T.; Ogawa, Y.; Tomeno, W.; Imajo, K.; Fujita, K.; Yoneda, M.; Takizawa, T.; Saito, S.; Nagashima, Y.; et al. Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, improves the pathogenesis in a rodent model of nonalcoholic steatohepatitis. Sci. Rep. 2017, 7, 42477. [Google Scholar] [CrossRef]
- Raza-Iqbal, S.; Tanaka, T.; Anai, M.; Inagaki, T.; Matsumura, Y.; Ikeda, K.; Taguchi, A.; Gonzalez, F.J.; Sakai, J.; Kodama, T. Transcriptome analysis of K-877 (a novel selective PPARα modulator (SPPARMα))-regulated genes in primary human hepatocytes and the mouse liver. J. Atheroscler. Thromb. 2015, 22, 28720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, M.; Kimura, T.; Yazaki, M.; Tanaka, N.; Yang, Y.; Nakajima, T.; Horiuchi, A.; Fang, Z.-Z.; Joshita, S.; Matsumoto, A. Steatogenesis in adult-onset type II citrullinemia is associated with down-regulation of PPARα. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 473–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simha, V. Management of hypertriglyceridemia. BMJ 2020, 371, m3109. [Google Scholar] [CrossRef]
- Kimura, T.; Shinji, A.; Horiuchi, A.; Tanaka, N.; Nagaya, T.; Shigeno, T.; Nakamura, N.; Komatsu, M.; Umemura, T.; Arakura, N.; et al. Clinical characteristics of young-onset ischemic colitis. Dig. Dis. Sci. 2012, 57, 1652–1659. [Google Scholar] [CrossRef]
- Kimura, T.; Shinji, A.; Tanaka, N.; Koinuma, M.; Yamaura, M.; Nagaya, T.; Joshita, S.; Komatsu, M.; Umemura, T.; Horiuchi, A.; et al. Association between lower air pressure and the onset of ischemic colitis: A case-control study. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1071–1078. [Google Scholar] [CrossRef]
- Ikeda, K.; Saitoh, S.; Kobayashi, M.; Suzuki, Y.; Tsubota, A.; Suzuki, F.; Arase, Y.; Murashima, N.; Chayama, K.; Kumada, H. Distinction between chronic hepatitis and liver cirrhosis in patients with hepatitis C virus infection. Practical discriminant function using common laboratory data. Hepatol. Res. 2000, 18, 252–266. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Wai, C.-T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.-F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Fujimori, N.; Kimura, T.; Tanaka, N.; Yamazaki, T.; Okumura, T.; Kobayashi, H.; Wakabayashi, S.I.; Yamashita, Y.; Sugiura, A.; Pham, J.; et al. 2-Step PLT16-AST44 method: Simplified liver fibrosis detection system in patients with non-alcoholic fatty liver disease. Hepatol. Res. 2022, 52, 352–363. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease: Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Seko, Y.; Sumida, Y.; Tanaka, S.; Mori, K.; Taketani, H.; Ishiba, H.; Hara, T.; Okajima, A.; Yamaguchi, K.; Moriguchi, M.; et al. Serum alanine aminotransferase predicts the histological course of non-alcoholic steatohepatitis in Japanese patients. Hepatol. Res. 2015, 45, E53–E61. [Google Scholar] [CrossRef] [PubMed]
- Nogami, A.; Yoneda, M.; Kobayashi, T.; Kessoku, T.; Honda, Y.; Ogawa, Y.; Suzuki, K.; Tomeno, W.; Imajo, K.; Kirikoshi, H.; et al. Assessment of 10-year changes in liver stiffness using vibration-controlled transient elastography in non-alcoholic fatty liver disease. Hepatol. Res. 2019, 49, 872–880. [Google Scholar] [CrossRef]
- Seko, Y.; Yamaguchi, K.; Umemura, A.; Yano, K.; Takahashi, A.; Okishio, S.; Kataoka, S.; Okuda, K.; Moriguchi, M.; Okanoue, T.; et al. Effect of pemafibrate on fatty acid levels and liver enzymes in non-alcoholic fatty liver disease patients with dyslipidemia: A single-arm, pilot study. Hepatol. Res. 2020, 50, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, S.; Tahara, T.; Lefor, A.K.; Ogura, M. Pemafibrate decreases markers of hepatic inflammation in patients with non-alcoholic fatty liver disease. Clin. Exp. Hepatol. 2020, 6, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, S.; Tahara, T.; Lefor, A.K.; Ogura, M. Pemafibrate improves hepatic inflammation, function and fibrosis in patients with non-alcoholic fatty liver disease: A one-year observational study. Clin. Exp. Hepatol. 2021, 7, 172–177. [Google Scholar] [CrossRef]
- Hatanaka, T.; Kakizaki, S.; Saito, N.; Nakano, Y.; Nakano, S.; Hazama, Y.; Yoshida, S.; Hachisu, Y.; Tanaka, Y.; Kashiwabara, K.; et al. Impact of Pemafibrate in Patients with Hypertriglyceridemia and Metabolic Dysfunction-associated Fatty Liver Disease Pathologically Diagnosed with Non-alcoholic Steatohepatitis: A Retrospective, Single-arm Study. Intern. Med. 2021, 60, 2167–2174. [Google Scholar] [CrossRef]
- Hatanaka, T.; Kosone, T.; Saito, N.; Takakusagi, S.; Tojima, H.; Naganuma, A.; Takagi, H.; Uraoka, T.; Kakizaki, S. Effect of 48-week pemafibrate on non-alcoholic fatty liver disease with hypertriglyceridemia, as evaluated by the FibroScan-aspartate aminotransferase score. JGH Open 2021, 5, 1183–1189. [Google Scholar] [CrossRef]
- Ikeda, S.; Sugihara, T.; Kihara, T.; Matsuki, Y.; Nagahara, T.; Takata, T.; Kitao, S.; Okura, T.; Yamamoto, K.; Isomoto, H. Pemafibrate Ameliorates Liver Dysfunction and Fatty Liver in Patients with Non-Alcoholic Fatty Liver Disease with Hypertriglyceridemia: A Retrospective Study with the Outcome after a Mid-Term Follow-Up. Diagnostics 2021, 11, 2316. [Google Scholar] [CrossRef]
- Nakajima, A.; Eguchi, Y.; Yoneda, M.; Imajo, K.; Tamaki, N.; Suganami, H.; Nojima, T.; Tanigawa, R.; Iizuka, M.; Iida, Y. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2021, 54, 1263–1277. [Google Scholar] [CrossRef]
- Kob, R.; Bollheimer, L.C.; Bertsch, T.; Fellner, C.; Djukic, M.; Sieber, C.C.; Fischer, B.E. Sarcopenic obesity: Molecular clues to a better understanding of its pathogenesis? Biogerontology 2015, 16, 15–29. [Google Scholar] [CrossRef]
- Araki, M.; Nakagawa, Y.; Oishi, A.; Han, S.I.; Wang, Y.; Kumagai, K.; Ohno, H.; Mizunoe, Y.; Iwasaki, H.; Sekiya, M.; et al. The Peroxisome Proliferator-Activated Receptor α (PPARα) Agonist Pemafibrate Protects against Diet-Induced Obesity in Mice. Int. J. Mol. Sci. 2018, 19, 2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smati, S.; Polizzi, A.; Fougerat, A.; Ellero-Simatos, S.; Blum, Y.; Lippi, Y.; Regnier, M.; Laroyenne, A.; Huillet, M.; Arif, M.; et al. Integrative study of diet-induced mouse models of NAFLD identifies PPARα as a sexually dimorphic drug target. Gut 2022, 71, 807–821. [Google Scholar] [CrossRef] [PubMed]






| All Patients (n = 88) | All Patients (n = 88) | ||||
|---|---|---|---|---|---|
| Median (IQR)/n (%) | Median (IQR) | ||||
| Age (years) | 57 | (46–66) | Laboratory data | ||
| Man | 35 | (39.8) | Alb (g/dL) | 4.4 | (4.2–4.6) |
| Liver cirrhosis | 6 | (6.8) | AST (U/L) | 43 | (30–61) |
| ALT (U/L) | 56 | (37–85) | |||
| Body composition | ALP (U/L) | 241 | (198–325) | ||
| Body weight (kg) | 71.9 | (61–79.3) | GGTP (U/L) | 59 | (40–95) |
| BMI (kg/m2) | 27.2 | (25.2–30.3) | Ferritin (ng/mL) $ | 163 | (87–278) |
| Fat mass (%) # | 39 | (30.4–42.6) | APRI | 0.7 | (0.4–1.1) |
| Soft lean mass (%) # | 57.7 | (54–66) | FIB-4 | 1.21 | (0.89–2.45) |
| SKM mass (%) # | 33.1 | (30.5–38.9) | M2BPGi (COI) * | 0.81 | (0.47–1.24) |
| SMI (kg/m2) # | 7.4 | (6.6–8.5) | TG (mg/dL) | 197 | (153–288) |
| TC (mg/dL) | 209 | (173–233) | |||
| Complications | LDL (mg/dL) | 124 | (99–150) | ||
| Type 2 DM | 41 | (47%) | HDL (mg/dL) | 43 | (36–656) |
| Hypertension | 31 | (35%) | Fasting glucose (mg/dL) | 117 | (104–137) |
| Obesity (BMI ≥ 25) | 68 | (77%) | HbA1c (%) | 6.3 | (5.9–6.6) |
| Sarcopenia # | 29 | (50%) | Cre (mg/dL) | 0.75 | (0.65–0.89) |
| CK (U/L) | 102 | (76–174) | |||
| Author | Year | No. of Cases | Drug Dose (Twice Daily) | Study Design | Pre ALT (U/L) | 3M ALT (U/L) | 6M ALT (U/L) | 12M ALT (U/L) |
|---|---|---|---|---|---|---|---|---|
| Our study | 2022 | 88 | 0.1 mg | Retrospective | 56 * | NS | 38 | NS |
| Nakajima et al. [29] | 2021 | 58 | 0.2 mg | Double-blind RCT | 83 † | NS | 50 | 48 |
| Shinozaki et al. [25] | 2021 | 22 | 0.1 mg | Retrospective | 65 † | NS | NS | 30 |
| Ikeda et al. [28] | 2021 | 16 | 0.1–0.2 mg | Retrospective | 65 * | NS | NS | 28 |
| Hatanaka et al. [26] | 2021 | 31 | 0.1 mg | Retrospective | 49 * | 33 | 25 | 32 |
| Hatanaka et al. [27] | 2021 | 10 | 0.1 mg | Retrospective | 52 * | 32 | 23 | NS |
| Shinozaki et al. [24] | 2020 | 38 | 0.1 mg | Retrospective | 64 † | 42 | NS | NS |
| Seko et al. [23] | 2020 | 20 | 0.1 mg | Prospective | 75 † | 44 | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwadare, T.; Kimura, T.; Kunimoto, H.; Tanaka, N.; Wakabayashi, S.-i.; Yamazaki, T.; Okumura, T.; Kobayashi, H.; Yamashita, Y.; Sugiura, A.; et al. Higher Responsiveness for Women, High Transaminase Levels, and Fat Percentage to Pemafibrate Treatment for NAFLD. Biomedicines 2022, 10, 2806. https://doi.org/10.3390/biomedicines10112806
Iwadare T, Kimura T, Kunimoto H, Tanaka N, Wakabayashi S-i, Yamazaki T, Okumura T, Kobayashi H, Yamashita Y, Sugiura A, et al. Higher Responsiveness for Women, High Transaminase Levels, and Fat Percentage to Pemafibrate Treatment for NAFLD. Biomedicines. 2022; 10(11):2806. https://doi.org/10.3390/biomedicines10112806
Chicago/Turabian StyleIwadare, Takanobu, Takefumi Kimura, Hideo Kunimoto, Naoki Tanaka, Shun-ichi Wakabayashi, Tomoo Yamazaki, Taiki Okumura, Hiroyuki Kobayashi, Yuki Yamashita, Ayumi Sugiura, and et al. 2022. "Higher Responsiveness for Women, High Transaminase Levels, and Fat Percentage to Pemafibrate Treatment for NAFLD" Biomedicines 10, no. 11: 2806. https://doi.org/10.3390/biomedicines10112806