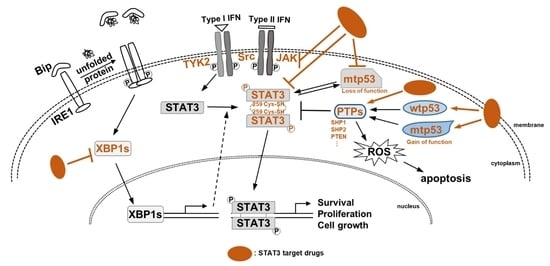
Special Issue “Role of STAT3 in Oncogenesis”
Funding
Acknowledgments
Conflicts of Interest
References
- Garg, M.; Shanmugam, M.K.; Bhardwaj, V.; Goel, A.; Gupta, R.; Sharma, A.; Baligar, P.; Kumar, A.P.; Goh, B.C.; Wang, L.; et al. The pleiotropic role of transcription factor STAT3 in oncogenesis and its targeting through natural products for cancer prevention and therapy. Med. Res. Rev. 2020, 41, 1291–1336. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chiang, S.Y.; Nam, D.; Chung, W.S.; Lee, J.; Na, Y.S.; Sethi, G.; Ahn, K.S. Capillarisin inhibits constitutive and inducible STAT3 activation through induction of SHP-1 and SHP-2 tyrosine phosphatases. Cancer Lett. 2014, 345, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, J.H.; Sethi, G.; Kim, C.; Baek, S.H.; Nam, D.; Chung, W.S.; Kim, S.H.; Shim, B.S.; Ahn, K.S. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014, 354, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Kim, S.H.; Sethi, G.; Ahn, K.S. Farnesol inhibits tumor growth and enhances the anticancer effects of bortezomib in multiple myeloma xenograft mouse model through the modulation of STAT3 signaling pathway. Cancer Lett. 2015, 360, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ahn, K.S.; Kim, C.; Shanmugam, M.K.; Siveen, K.S.; Arfuso, F.; Samym, R.P.; Deivasigamanim, A.; Lim, L.H.; Wang, L.; et al. Nimbolide-Induced Oxidative Stress Abrogates STAT3 Signaling Cascade and Inhibits Tumor Growth in Transgenic Adenocarcinoma of Mouse Prostate Model. Antioxid. Redox Signal 2016, 24, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Byun, W.S.; Bae, E.S.; Cui, J.; Park, H.J.; Oh, D.C.; Lee, S.K. Antitumor Activity of Pulvomycin via Targeting Activated-STAT3 Signaling in Docetaxel-Resistant Triple-Negative Breast Cancer Cells. Biomedicines 2021, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Ha, I.J.; Um, J.Y.; Ahn, K.S. Albendazole Exhibits Anti-Neoplastic Actions against Gastric Cancer Cells by Affecting STAT3 and STAT5 Activation by Pleiotropic Mechanism(s). Biomedicines 2021, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Bauvois, B.; Pramil, E.; Jondreville, L.; Quiney, C.; Nguyen-Khac, F.; Susin, S.A. Activation of Interferon Signaling in Chronic Lymphocytic Leukemia Cells Contributes to Apoptosis Resistance via a JAK-Src/STAT3/Mcl-1 Signaling Pathway. Biomedicines 2021, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Gonnella, R.; Gilardini Montani, M.S.; Guttieri, L.; Romeo, M.A.; Santarelli, R.; Cirone, M. IRE1 Alpha/XBP1 Axis Sustains Primary Effusion Lymphoma Cell Survival by Promoting Cytokine Release and STAT3 Activation. Biomedicines 2021, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Kim, J.T.; Kim, S.J.; Cho, N.C.; Kim, K.; Lee, S.; Suh, Y.G.; Cho, K.C.; Kim, K.P.; Surh, Y.J. An Electrophilic Deguelin Analogue Inhibits STAT3 Signaling in H-Ras-Transformed Human Mammary Epithelial Cells: The Cysteine 259 Residue as a Potential Target. Biomedicines 2020, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Park, H.M.; Kim, J.; Hong, J.T.; Yoon, D.Y. STAT3 and p53: Dual Target for Cancer Therapy. Biomedicines 2020, 8, 637. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, K.S. Special Issue “Role of STAT3 in Oncogenesis”. Biomedicines 2022, 10, 2689. https://doi.org/10.3390/biomedicines10112689
Ahn KS. Special Issue “Role of STAT3 in Oncogenesis”. Biomedicines. 2022; 10(11):2689. https://doi.org/10.3390/biomedicines10112689
Chicago/Turabian StyleAhn, Kwang Seok. 2022. "Special Issue “Role of STAT3 in Oncogenesis”" Biomedicines 10, no. 11: 2689. https://doi.org/10.3390/biomedicines10112689