Recent Advances in Electrochemiluminescence Emitters for Biosensing and Imaging of Protein Biomarkers
Abstract
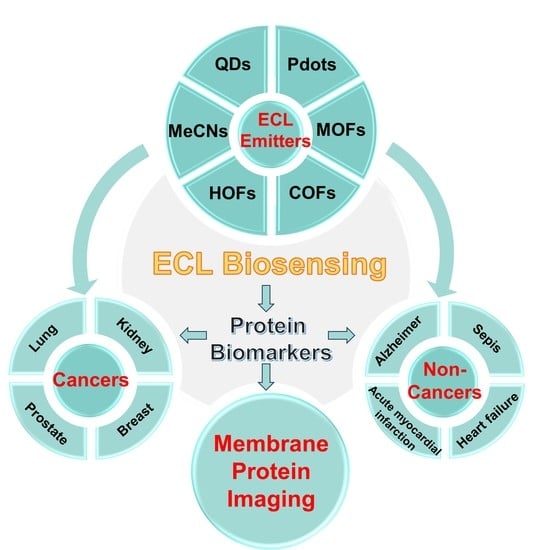
:1. Introduction
2. Nanomaterial-Based ECL Emitters
2.1. Quantum Dot-Based ECL Emitters

2.2. Metal Nanocluster-Based ECL Emitters
2.3. MOFs-Based ECL Emitters
2.4. COFs/HOF-Based ECL Emitters
2.5. Polymer Dot-Based ECL Emitters
3. Application of ECL Emitters for the Analysis of Protein-Based Biomarkers
3.1. Protein-Based Cancer Biomarkers
3.2. Protein-Based Non-Cancer Biomarkers
3.3. ECL Imaging
4. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, L.; Xu, G. Applications and trends in electrochemiluminescence. Chem. Soc. Rev. 2010, 39, 3275–3304. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hang, J.; Qu, W.; Wang, Y.; Wang, L.; Zhou, P.; Ding, H.; Su, B.; Lei, J.; Guo, W.; et al. Gold Microbeads Enabled Proximity Electrochemiluminescence for Highly Sensitive and Size-Encoded Multiplex Immunoassays. J. Am. Chem. Soc. 2023, 145, 16026–16036. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, J.; Zhou, P.; Liu, J.; Qiao, Z.; Yu, K.; Jiang, J.; Su, B. Electrochemiluminescence Distance and Reactivity of Coreactants Determine the Sensitivity of Bead-Based Immunoassays. Angew. Chem. Int. Ed. 2023, 62, e202216525. [Google Scholar] [CrossRef]
- Miao, W. Electrogenerated Chemiluminescence and Its Biorelated Applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef]
- Voci, S.; Goudeau, B.; Valenti, G.; Lesch, A.; Jović, M.; Rapino, S.; Paolucci, F.; Arbault, S.; Sojic, N. Surface-Confined Electrochemiluminescence Microscopy of Cell Membranes. J. Am. Chem. Soc. 2018, 140, 14753–14760. [Google Scholar] [CrossRef] [PubMed]
- Visco, R.E.; Chandross, E.A. Electroluminescence in Solutions of Aromatic Hydrocarbons. J. Am. Chem. Soc. 1964, 86, 5350–5351. [Google Scholar] [CrossRef]
- Han, Q.; Wang, C.; Liu, P.; Zhang, G.; Song, L.; Fu, Y. Three kinds of porphyrin dots as near-infrared electrochemiluminescence luminophores: Facile synthesis and biosensing. Chem. Eng. J. 2021, 421, 129761. [Google Scholar] [CrossRef]
- Nasiri Khonsari, Y.; Sun, S. Recent trends in electrochemiluminescence aptasensors and their applications. Chem. Commun. 2017, 53, 9042–9054. [Google Scholar] [CrossRef]
- Li, Z.; Huang, X.; Liu, H.; Luo, F.; Qiu, B.; Lin, Z.; Chen, H. Electrochemiluminescence Biosensor for Hyaluronidase Based on the Adjustable Electrostatic Interaction between the Surface-Charge-Controllable Nanoparticles and Negatively Charged Electrode. ACS Sens. 2022, 7, 2012–2019. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Li, B.; Liu, J.; Jiang, D.; Liu, B.; Sojic, N. Single Biomolecule Imaging by Electrochemiluminescence. J. Am. Chem. Soc. 2021, 143, 17910–17914. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, R.; Chai, Y.; Zhuo, Y.; Mao, L.; Yuan, S. Ru(bpy)32+-doped silica nanoparticles labeling for a sandwich-type electrochemiluminescence immunosensor. Biosens. Bioelectron. 2010, 25, 1851–1855. [Google Scholar] [CrossRef]
- Dong, Y.-P.; Chen, G.; Zhou, Y.; Zhu, J.-J. Electrochemiluminescent Sensing for Caspase-3 Activity Based on Ru(bpy)32+-Doped Silica Nanoprobe. Anal. Chem. 2016, 88, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.; Rampazzo, E.; Bonacchi, S.; Petrizza, L.; Marcaccio, M.; Montalti, M.; Prodi, L.; Paolucci, F. Variable Doping Induces Mechanism Swapping in Electrogenerated Chemiluminescence of Ru(bpy)32+ Core–Shell Silica Nanoparticles. J. Am. Chem. Soc. 2016, 138, 15935–15942. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Li, X.; Fang, J.; Li, M.; Zhang, J.; Zhao, G.; Cao, W.; Wei, Q. Oxygen Free Radical Scavenger PtPd@PDA as a Dual-Mode Quencher of Electrochemiluminescence Immunosensor for the Detection of AFB1. Anal. Chem. 2022, 94, 11476–11482. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiang, Y.; Li, J.; Kong, Q.; Zhai, H.; Xu, R.; Yang, F.; Sun, X.; Guo, Y. A novel electrochemiluminescence aptasensor based on copper-gold bimetallic nanoparticles and its applications. Biosens. Bioelectron. 2021, 194, 113601. [Google Scholar] [CrossRef]
- Lin, Y.; Cao, J.; Li, X.; Zhang, X.; Zhang, J.; Lin, Z. A novel molecularly imprinted electrochemiluminescence sensor based on a Ru(bpy)32+/MWCNTs/nano-TiO2-Nafion electrode for the detection of bisphenol A. Anal. Methods 2016, 8, 7445–7452. [Google Scholar] [CrossRef]
- Ju, J.; Ding, Q.; Xie, J.; Li, G. Label-free electrochemiluminescence immunosensor for mucoprotein 1 using a graphene oxide-Ru(Bpy)32+-polyaniline nanocomposite. Anal. Lett. 2023, 1–12. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Lu, L.; Sun, Z.; Zhang, Y.; Dang, F.; Qian, L. Porous graphene containing immobilized Ru(II) tris-bipyridyl for use in electrochemiluminescence sensing of tripropylamine. Microchim. Acta 2016, 183, 1211–1217. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, G.; Ni, J.; Wang, Q.; Lin, Z. From signal amplification to restrained background: Magnetic graphene oxide assisted homogeneous electrochemiluminescence aptasensor for highly sensitive detection of okadaic acid. Sens. Actuators B Chem. 2021, 327, 128872. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Xi, J.; Zhang, A.; Chen, Y.; Lu, F.; Chen, Z. A novel electrochemiluminescence sensor based on Ru(bpy)32+ immobilized by graphene on glassy carbon electrode surface via in situ wet-chemical reaction. Sens. Actuators B Chem. 2012, 171–172, 1159–1165. [Google Scholar] [CrossRef]
- Yao, B.; Zhang, J.; Fan, Z.; Ding, Y.; Zhou, B.; Yang, R.; Zhao, J.; Zhang, K. Rational Engineering of the DNA Walker Amplification Strategy by Using a Au@Ti3C2@PEI-Ru(dcbpy)32+ Nanocomposite Biosensor for Detection of the SARS-CoV-2 RdRp Gene. ACS Appl. Mater. Interfaces 2021, 13, 19816–19824. [Google Scholar] [CrossRef]
- Xing, H.; Xia, H.; Fan, Y.; Xue, Y.; Peng, C.; Ren, J.; Li, J.; Wang, E. A Solid-State Electrochemiluminescence Sensor Based on Novel Two-Dimensional Ti3C2 MXene. ChemElectroChem 2021, 8, 1858–1863. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, X.; Chen, T.; Xu, G.; Liu, M.; Liu, J.; Xu, Y. Two-dimensional titanium carbide (MXene)-based solid-state electrochemiluminescent sensor for label-free single-nucleotide mismatch discrimination in human urine. Sens. Actuators B Chem. 2018, 263, 400–407. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, D.; Wu, G.; Wang, H.; Ru, F.; Zhang, X.; Li, L.; Qian, Y.; Lu, X. A novel “dual-potential” ratiometric electrochemiluminescence DNA sensor based on enhancing and quenching effect by G-quadruplex/hemin and Au-Luminol bifunctional nanoparticles. Biosens. Bioelectron. 2018, 106, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ren, X.; Ai, Y.; Li, M.; Zhang, B.; Zou, G. Luminophore-Surface-Engineering-Enabled Low-Triggering-Potential and Coreactant-Free Electrochemiluminescence for Protein Determination. Anal. Chem. 2023, 95, 6948–6954. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Du, J.; Luo, J.; Chen, S.; Yuan, R. Coreactant-free electrochemiluminescence biosensor for the determination of organophosphorus pesticides. Biosens. Bioelectron. 2020, 150, 111898. [Google Scholar] [CrossRef]
- Xie, J.; Yang, G.; Tan, X.; Yuan, R.; Chen, S. Coreactant-free electrochemiluminescence of polyfluorene nanoparticle coupling double quencher for β-amyloid1-42 detection. Talanta 2023, 258, 124398. [Google Scholar] [CrossRef]
- Song, X.; Zhao, L.; Luo, C.; Ren, X.; Wang, X.; Yang, L.; Wei, Q. Bioactivity-protective electrochemiluminescence sensor using CeO2/Co4N heterostructures as highly effective coreaction accelerators for ultrasensitive immunodetection. Sens. Actuators B Chem. 2022, 355, 131158. [Google Scholar] [CrossRef]
- Wu, F.-F.; Zhou, Y.; Zhang, H.; Yuan, R.; Chai, Y.-Q. Electrochemiluminescence Peptide-Based Biosensor with Hetero-Nanostructures as Coreaction Accelerator for the Ultrasensitive Determination of Tryptase. Anal. Chem. 2018, 90, 2263–2270. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Y.; Zhao, J.; He, Y.; Yuan, R.; Chen, S. Dual-emitting Iridium nanorods combining dual-regulating coreaction accelerator Ag nanoparticles for electrochemiluminescence ratio determination of amyloid-β oligomers. Biosens. Bioelectron. 2022, 216, 114629. [Google Scholar] [CrossRef]
- Ma, C.; Cao, Y.; Gou, X.; Zhu, J.-J. Recent Progress in Electrochemiluminescence Sensing and Imaging. Anal. Chem. 2020, 92, 431–454. [Google Scholar] [CrossRef]
- Husain, R.A.; Barman, S.R.; Chatterjee, S.; Khan, I.; Lin, Z.-H. Enhanced biosensing strategies using electrogenerated chemiluminescence: Recent progress and future prospects. J. Mater. Chem. B 2020, 8, 3192–3212. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, C. Electrogenerated Chemiluminescence Biosensing. Anal. Chem. 2020, 92, 524–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, G.S.; Ahmed, T.; Heiss, L.; Walia, S.; Bhaskaran, M.; Sriram, S. Rapid and Selective Biomarker Detection with Conductometric Sensors. Small 2021, 17, 2005582. [Google Scholar] [CrossRef]
- Liu, R.; Ye, X.; Cui, T. Recent Progress of Biomarker Detection Sensors. Research 2020, 2020, 7949037. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, X.; Zhang, W.; Jia, L.-P.; Ma, R.-N.; Jia, W.-L.; Wang, H.-S. A dual-potential electrochemiluminescence sensor for ratiometric detection of carcinoembryonic antigen based on single luminophor. Sens. Actuators B Chem. 2020, 325, 128776. [Google Scholar] [CrossRef]
- Luo, W.; Ye, Z.; Ma, P.; Wu, Q.; Song, D. Preparation of a disposable electrochemiluminescence sensor chip based on an MXene-loaded ruthenium luminescent agent and its application in the detection of carcinoembryonic antigens. Analyst 2022, 147, 1986–1994. [Google Scholar] [CrossRef]
- Shang, L.; Shi, B.-J.; Zhang, W.; Jia, L.-P.; Ma, R.-N.; Xue, Q.-W.; Wang, H.-S. Ratiometric Electrochemiluminescence Sensing of Carcinoembryonic Antigen Based on Luminol. Anal. Chem. 2022, 94, 12845–12851. [Google Scholar] [CrossRef]
- Huang, B.; Liu, X.-P.; Chen, J.-S.; Mao, C.; Niu, H.-L.; Jin, B.-K. Electrochemiluminescence immunoassay for the prostate-specific antigen by using a CdS/chitosan/g-C3N4 nanocomposite. Microchim. Acta 2020, 187, 155. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, D.; Kan, X. The combination of highly efficient resonance energy transfer in one nanocomposite and ferrocene-quenching for ultrasensitive electrochemiluminescence bioanalysis. Biosens. Bioelectron. 2022, 210, 114347. [Google Scholar] [CrossRef]
- Zheng, L.; Guo, Q.; Yang, C.; Wang, J.; Xu, X.; Nie, G. Electrochemiluminescence and photoelectrochemistry dual-signal immunosensor based on Ru(bpy)32+-functionalized MOF for prostate-specific antigen sensitive detection. Sens. Actuators B Chem. 2023, 379, 133269. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Zhang, R.; Yan, F. Dual-mode electrochemiluminescence and electrochemical sensor for alpha-fetoprotein detection in human serum based on vertically ordered mesoporous silica films. Front. Chem. 2022, 10, 1023998. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Jiang, X.; Mo, G.; Feng, J.; Deng, B. Boron nitride quantum dots as electrochemiluminescence coreactants of rGO@Au@Ru–SiO2 for label-free detection of AFP in human serum. Electrochim. Acta 2020, 335, 135621. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, Y.; Zhang, Q.; Guo, Y.; Ma, Q. The high luminescent polydopamine nanosphere-based ECL biosensor with steric effect for MUC1 detection. Chem. Eng. J. 2020, 385, 123825. [Google Scholar] [CrossRef]
- Huang, W.; Hu, G.-B.; Yao, L.-Y.; Yang, Y.; Liang, W.-B.; Yuan, R.; Xiao, D.-R. Matrix Coordination-Induced Electrochemiluminescence Enhancement of Tetraphenylethylene-Based Hafnium Metal–Organic Framework: An Electrochemiluminescence Chromophore for Ultrasensitive Electrochemiluminescence Sensor Construction. Anal. Chem. 2020, 92, 3380–3387. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Liang, W.-B.; Hu, G.-B.; Yao, L.-Y.; Yang, Y.; Zhou, K.; Yuan, R.; Xiao, D.-R. Two Birds with One Stone: Surface Functionalization and Delamination of Multilayered Ti3C2Tx MXene by Grafting a Ruthenium(II) Complex to Achieve Conductivity-Enhanced Electrochemiluminescence. Anal. Chem. 2021, 93, 1834–1841. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, B.; Wang, C.; Fan, D.; Liu, X.; Wei, Q.; Ju, H.; Wu, D. Dual-strategy ECL biosensor based on rare Eu(II,III)-MOF as probe with antenna effect and sensitization for CYFRA 21-1 trace analysis. Sens. Actuators B Chem. 2023, 377, 133101. [Google Scholar] [CrossRef]
- Jian, L.; Wang, X.; Hao, L.; Liu, Y.; Yang, H.; Zheng, X.; Feng, W. Electrochemiluminescence immunosensor for cytokeratin fragment antigen 21-1 detection using electrochemically mediated atom transfer radical polymerization. Microchim. Acta 2021, 188, 115. [Google Scholar] [CrossRef]
- Xue, J.; Yang, L.; Du, Y.; Ren, Y.; Ren, X.; Ma, H.; Wu, D.; Ju, H.; Li, Y.; Wei, Q. Electrochemiluminescence sensing platform based on functionalized poly-(styrene-co-maleicanhydride) nanocrystals and iron doped hydroxyapatite for CYFRA 21-1 immunoassay. Sens. Actuators B Chem. 2020, 321, 128454. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Cai, R.; Tan, W. Ultrahighly Sensitive Sandwich-Type Electrochemical Immunosensor for Selective Detection of Tumor Biomarkers. ACS Appl. Mater. Interfaces 2022, 14, 44222–44227. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Xu, R.; Wang, H.; Zhang, Y.; Wei, Q. Progress and Prospects of Electrochemiluminescence Biosensors Based on Porous Nanomaterials. Biosensors 2022, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Wang, B.; Liu, X.; Lu, C. Understanding role of microstructures of nanomaterials in electrochemiluminescence properties and their applications. TrAC Trends Anal. Chem. 2023, 162, 117030. [Google Scholar] [CrossRef]
- Padmakumari Kurup, C.; Abdullah Lim, S.; Ahmed, M.U. Nanomaterials as signal amplification elements in aptamer-based electrochemiluminescent biosensors. Bioelectrochemistry 2022, 147, 108170. [Google Scholar] [CrossRef]
- Zou, G.; Ju, H. Electrogenerated Chemiluminescence from a CdSe Nanocrystal Film and Its Sensing Application in Aqueous Solution. Anal. Chem. 2004, 76, 6871–6876. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Zhang, C. Toward Biocompatible Semiconductor Quantum Dots: From Biosynthesis and Bioconjugation to Biomedical Application. Chem. Rev. 2015, 115, 11669–11717. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Miao, W.; Zou, G. Molecular-Counting-Free and Electrochemiluminescent Single-Molecule Immunoassay with Dual-Stabilizers-Capped CdSe Nanocrystals as Labels. Anal. Chem. 2016, 88, 5482–5488. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Yu, Y.; Zou, G. A Monochromatic Electrochemiluminescence Sensing Strategy for Dopamine with Dual-Stabilizers-Capped CdSe Quantum Dots as Emitters. Anal. Chem. 2014, 86, 2784–2788. [Google Scholar] [CrossRef]
- Lin, D.; Wu, J.; Yan, F.; Deng, S.; Ju, H. Ultrasensitive Immunoassay of Protein Biomarker Based on Electrochemiluminescent Quenching of Quantum Dots by Hemin Bio-Bar-Coded Nanoparticle Tags. Anal. Chem. 2011, 83, 5214–5221. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Z.; Wang, J.; Cui, C.; Hu, L. An “off–on” electrochemiluminescence aptasensor for determination of lincomycin based on CdS QDs/carboxylated g-C3N4. Microchim. Acta 2023, 190, 11. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Z.; Liu, J.; Liu, H.; Bian, W.; Tian, D.; Xia, F.; Zhou, C. A novel electrochemiluminescence aptasensor based CdTe QDs@NH2-MIL-88(Fe) for signal amplification. Electrochimica Acta 2020, 354, 136644. [Google Scholar] [CrossRef]
- Zhu, H.-Y.; Ding, S.-N. Dual-signal-amplified electrochemiluminescence biosensor for microRNA detection by coupling cyclic enzyme with CdTe QDs aggregate as luminophor. Biosens. Bioelectron. 2019, 134, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, Y.; Tan, K.; Yang, J.; Chen, S.; Yuan, R. Novel Ratiometric Electrochemiluminescence Biosensor Based on BP-CdTe QDs with Dual Emission for Detecting MicroRNA-126. Anal. Chem. 2021, 93, 12400–12408. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, H.; Jiang, X.; Huang, L.; Chen, L.; Li, N. Quantum Dot-Based Near-Infrared Electrochemiluminescent Immunosensor with Gold Nanoparticle-Graphene Nanosheet Hybrids and Silica Nanospheres Double-Assisted Signal Amplification. Anal. Chem. 2012, 84, 4893–4899. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, Z.; Wu, X.; Chen, H.; Chai, Y.; Yuan, R. Highly Efficient Electrochemiluminescence Resonance Energy Transfer System in One Nanostructure: Its Application for Ultrasensitive Detection of MicroRNA in Cancer Cells. Anal. Chem. 2017, 89, 6029–6035. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, F.; Ge, S.; Zhang, L.; Zhang, Z.; Liu, Y.; Zhang, Y.; Ge, S.; Yu, J. Programmable T-Junction Structure-Assisted CRISPR/Cas12a Electrochemiluminescence Biosensor for Detection of Sa-16S rDNA. ACS Appl. Mater. Interfaces 2023, 15, 617–625. [Google Scholar] [CrossRef]
- Yang, E.; Zhang, Y.; Shen, Y. Quantum dots for electrochemiluminescence bioanalysis—A review. Anal. Chim. Acta 2022, 1209, 339140. [Google Scholar] [CrossRef]
- Pan, D.; Chen, K.; Zhou, Q.; Zhao, J.; Xue, H.; Zhang, Y.; Shen, Y. Engineering of CdTe/SiO2 nanocomposites: Enhanced signal amplification and biocompatibility for electrochemiluminescent immunoassay of alpha-fetoprotein. Biosens. Bioelectron. 2019, 131, 178–184. [Google Scholar] [CrossRef]
- Shen, C.; Li, Y.; Li, Y.; Wang, S.; Li, Y.; Tang, F.; Wang, P.; Liu, H.; Li, Y.; Liu, Q. A double reaction system induced electrochemiluminescence enhancement based on SnS2 QDs@MIL-101 for ultrasensitive detection of CA242. Talanta 2022, 247, 123575. [Google Scholar] [CrossRef]
- Lei, Y.-M.; Zhou, J.; Chai, Y.-Q.; Zhuo, Y.; Yuan, R. SnS2 Quantum Dots as New Emitters with Strong Electrochemiluminescence for Ultrasensitive Antibody Detection. Anal. Chem. 2018, 90, 12270–12277. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, A.-Y.; Huang, D.; Chai, Y.-Q.; Zhuo, Y.; Yuan, R. MoS2 Quantum Dots as New Electrochemiluminescence Emitters for Ultrasensitive Bioanalysis of Lipopolysaccharide. Anal. Chem. 2017, 89, 8335–8342. [Google Scholar] [CrossRef]
- Hua, Q.; Tang, F.; Wang, X.; Li, M.; Gu, X.; Sun, W.; Luan, F.; Tian, C.; Zhuang, X. Electrochemiluminescence sensor based on EuS nanocrystals for ultrasensitive detection of mercury ions in seafood. Sens. Actuators B Chem. 2022, 352, 131075. [Google Scholar] [CrossRef]
- Jiang, D.; Wei, M.; Du, X.; Qin, M.; Shan, X.; Wang, W.; Chen, Z. Ultrasensitive near-infrared aptasensor for enrofloxacin detection based on wavelength tunable AgBr nanocrystals electrochemiluminescence emission triggered by O-terminated Ti3C2 MXene. Biosens. Bioelectron. 2022, 200, 113917. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.-M.; Zhuo, Y.; Guo, M.-L.; Chai, Y.-Q.; Yuan, R. Pore Confinement-Enhanced Electrochemiluminescence on SnO2 Nanocrystal Xerogel with NO3– As Co-Reactant and Its Application in Facile and Sensitive Bioanalysis. Anal. Chem. 2020, 92, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Du, Y.; Niu, X.; Li, G.; Zhu, D.; Yu, Q.; Zou, G.; Ju, H. Arginine-modified black phosphorus quantum dots with dual excited states for enhanced electrochemiluminescence in bioanalysis. Nat. Commun. 2022, 13, 7302. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Liu, X.; Chen, X.; Wang, L.; Yang, W. Black phosphorus quantum dots as novel electrogenerated chemiluminescence emitters for the detection of Cu2+. Chem. Commun. 2020, 56, 4680–4683. [Google Scholar] [CrossRef]
- Li, P.; Yu, J.; Zhao, K.; Deng, A.; Li, J. Efficient enhancement of electrochemiluminescence from tin disulfide quantum dots by hollow titanium dioxide spherical shell for highly sensitive detection of chloramphenicol. Biosens. Bioelectron. 2020, 147, 111790. [Google Scholar] [CrossRef]
- Fu, L.; Fu, K.; Gao, X.; Dong, S.; Zhang, B.; Fu, S.; Hsu, H.-Y.; Zou, G. Enhanced Near-Infrared Electrochemiluminescence from Trinary Ag–In–S to Multinary Ag–Ga–In–S Nanocrystals via Doping-in-Growth and Its Immunosensing Applications. Anal. Chem. 2021, 93, 2160–2165. [Google Scholar] [CrossRef]
- Long, X.; Zhang, F.; He, Y.; Hou, S.; Zhang, B.; Zou, G. Promising Anodic Electrochemiluminescence of Nontoxic Core/Shell CuInS2/ZnS Nanocrystals in Aqueous Medium and Its Biosensing Potential. Anal. Chem. 2018, 90, 3563–3569. [Google Scholar] [CrossRef]
- Wang, C.; Liu, L.; Liu, X.; Chen, Y.; Wang, X.; Fan, D.; Kuang, X.; Sun, X.; Wei, Q.; Ju, H. Highly-sensitive electrochemiluminescence biosensor for NT-proBNP using MoS2@Cu2S as signal-enhancer and multinary nanocrystals loaded in mesoporous UiO-66-NH2 as novel luminophore. Sens. Actuators B Chem. 2020, 307, 127619. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, H.; Shen, Y.; Hu, N.; Shi, W. Nitrogen-doped Ti3C2 MXene quantum dots as novel high-efficiency electrochemiluminescent emitters for sensitive mucin 1 detection. Sens. Actuators B Chem. 2022, 350, 130891. [Google Scholar] [CrossRef]
- Chen, A.; Liang, W.; Wang, H.; Zhuo, Y.; Chai, Y.; Yuan, R. Anodic Electrochemiluminescence of Carbon Dots Promoted by Nitrogen Doping and Application to Rapid Cancer Cell Detection. Anal. Chem. 2020, 92, 1379–1385. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Zhang, R.; He, S.; Ding, Z.; Ding, L. Electrochemiluminescence of water-dispersed nitrogen and sulfur doped carbon dots synthesized from amino acids. Analyst 2021, 146, 5287–5293. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Yuan, R.; Chai, Y. Fluorine-nitrogen co-doped carbon dots with stable and strong electrochemiluminescence as an emitter for ultrasensitive detection of HIV-DNA fragment. Sens. Actuators B Chem. 2023, 379, 133260. [Google Scholar] [CrossRef]
- Yang, E.; Ning, Z.; Yin, F.; Fang, Z.; Chen, M.; Zhang, M.; Xu, W.; Zhang, Y.; Shen, Y. Surface plasmon-enhanced electrochemiluminescence of P, N-doped carbon dots for ultrasensitive detection of BRAF gene. Sens. Actuators B Chem. 2022, 369, 132288. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, L.; Yin, B.; Du, W.; Wang, X.; Liu, Y.; Chen, S.; Zhu, M. Bright Near-Infrared Circularly Polarized Electrochemiluminescence from Au9Ag4 Nanoclusters. Chem. Sci. 2023, 14, 7304–7309. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, J.-L.; Ma, Y.; Zhou, Y.; Zhu, J.-J. Recent progress of metal nanoclusters in electrochemiluminescence. Dalton Trans. 2022, 51, 8927–8937. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhao, Y.; Zhang, Z.; Xu, G. Recent Advances in Electrochemiluminescence and Chemiluminescence of Metal Nanoclusters. Molecules 2020, 25, 5208. [Google Scholar] [CrossRef] [PubMed]
- Díez, I.; Pusa, M.; Kulmala, S.; Jiang, H.; Walther, A.; Goldmann, A.S.; Müller, A.H.E.; Ikkala, O.; Ras, R.H.A. Color Tunability and Electrochemiluminescence of Silver Nanoclusters. Angew. Chem. Int. Ed. 2009, 48, 2122–2125. [Google Scholar] [CrossRef]
- Wang, T.; Wang, D.; Padelford, J.W.; Jiang, J.; Wang, G. Near-Infrared Electrogenerated Chemiluminescence from Aqueous Soluble Lipoic Acid Au Nanoclusters. J. Am. Chem. Soc. 2016, 138, 6380–6383. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Shen, Y.; Zhang, J.; Zhu, J.-J. Electrogenerated Chemiluminescence of Au Nanoclusters for the Detection of Dopamine. Anal. Chem. 2011, 83, 661–665. [Google Scholar] [CrossRef]
- Fang, Y.-M.; Song, J.; Li, J.; Wang, Y.-W.; Yang, H.-H.; Sun, J.-J.; Chen, G.-N. Electrogenerated chemiluminescence from Au nanoclusters. Chem. Commun. 2011, 47, 2369–2371. [Google Scholar] [CrossRef] [PubMed]
- Hesari, M.; Ding, Z. Efficient Near-Infrared Electrochemiluminescence from Au18 Nanoclusters. Chem. Eur. J. 2021, 27, 14821–14825. [Google Scholar] [CrossRef] [PubMed]
- Hesari, M.; Ding, Z. Identifying Highly Photoelectrochemical Active Sites of Two Au21 Nanocluster Isomers toward Bright Near-Infrared Electrochemiluminescence. J. Am. Chem. Soc. 2021, 143, 19474–19485. [Google Scholar] [CrossRef] [PubMed]
- Hesari, M.; Ma, H.; Ding, Z. Monitoring single Au38 nanocluster reactions via electrochemiluminescence. Chem. Sci. 2021, 12, 14540–14545. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, X.; Shao, N.; Zhang, G.; Li, S.; Zhou, H.; Wu, J.; Tian, Y. Crystal structure, optical properties and electrochemiluminescence of Cu(I), Ag(I) and Au(I) complexes that contain the cyanoacetic acid triphenylamine ligand. Polyhedron 2015, 93, 17–22. [Google Scholar] [CrossRef]
- Zhu, M.-J.; Pan, J.-B.; Wu, Z.-Q.; Gao, X.-Y.; Zhao, W.; Xia, X.-H.; Xu, J.-J.; Chen, H.-Y. Electrogenerated Chemiluminescence Imaging of Electrocatalysis at a Single Au-Pt Janus Nanoparticle. Angew. Chem. Int. Ed. 2018, 57, 4010–4014. [Google Scholar] [CrossRef]
- Qian, H.; Jiang, D.; Li, G.; Gayathri, C.; Das, A.; Gil, R.R.; Jin, R. Monoplatinum Doping of Gold Nanoclusters and Catalytic Application. J. Am. Chem. Soc. 2012, 134, 16159–16162. [Google Scholar] [CrossRef]
- Chen, S.; Ma, H.; Padelford, J.W.; Qinchen, W.; Yu, W.; Wang, S.; Zhu, M.; Wang, G. Near Infrared Electrochemiluminescence of Rod-Shape 25-Atom AuAg Nanoclusters That Is Hundreds-Fold Stronger Than That of Ru(bpy)3 Standard. J. Am. Chem. Soc. 2019, 141, 9603–9609. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J.; Xiong, C.; Xiao, Y.; Zhang, X.; Wang, S. Enhanced electrochemiluminescence of gold nanoclusters via silver doping and their application for ultrasensitive detection of dopamine. Analyst 2019, 144, 2643–2648. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, L.; Wu, J.; Wu, Y.; Liu, Y.; Du, J.; Lu, X. A Highly Sensitive Electrochemiluminescence Spermine Biosensor Based on Au−Ag Bimetallic Nanoclusters. Electroanalysis 2021, 33, 2016–2024. [Google Scholar] [CrossRef]
- Nie, Y.; Tao, X.; Zhang, H.; Chai, Y.; Yuan, R. Self-Assembly of Gold Nanoclusters into a Metal–Organic Framework with Efficient Electrochemiluminescence and Their Application for Sensitive Detection of Rutin. Anal. Chem. 2021, 93, 3445–3451. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xiao, H.; Yang, S.; Liu, C.; Liang, J.; Tang, Y. Ultrasensitive detection of pentachlorophenol based on enhanced electrochemiluminescence of Au nanoclusters/graphene hybrids. Sens. Actuators B Chem. 2014, 194, 325–331. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, W.; Wang, Q.; Xiao, H.; Kuang, Y.; Liu, C. Novel anodic electrochemiluminescence system of Pt nanocluster/graphene hybrids for ultrasensitive detection of Cu2+. J. Electroanal. Chem. 2016, 772, 73–79. [Google Scholar] [CrossRef]
- Guo, C.; Duan, F.; Zhang, S.; He, L.; Wang, M.; Chen, J.; Zhang, J.; Jia, Q.; Zhang, Z.; Du, M. Heterostructured hybrids of metal–organic frameworks (MOFs) and covalent–organic frameworks (COFs). J. Mater. Chem. A 2022, 10, 475–507. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Li, Z.; Goyal, N.; Du, T.; He, J.; Li, G.K. Shaping of Metal–Organic Frameworks: A Review. Energy Fuels 2022, 36, 2927–2944. [Google Scholar] [CrossRef]
- Hwang, J.; Ejsmont, A.; Freund, R.; Goscianska, J.; Schmidt, B.V.K.J.; Wuttke, S. Controlling the morphology of metal–organic frameworks and porous carbon materials: Metal oxides as primary architecture-directing agents. Chem. Soc. Rev. 2020, 49, 3348–3422. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Chi, H.; Yang, S.; Niu, Q.; Wu, D.; Cao, W.; Li, T.; Ma, H.; Wei, Q. Self-Luminescent Lanthanide Metal–Organic Frameworks as Signal Probes in Electrochemiluminescence Immunoassay. J. Am. Chem. Soc. 2021, 143, 504–512. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, G.; Li, X.; Fang, J.; Miao, J.; Wei, Q.; Cao, W. Electrochemiluminescence immunosensor of “signal-off” for β-amyloid detection based on dual metal-organic frameworks. Talanta 2020, 208, 120376. [Google Scholar] [CrossRef]
- Huang, W.; Hu, G.-B.; Liang, W.-B.; Wang, J.-M.; Lu, M.-L.; Yuan, R.; Xiao, D.-R. Ruthenium(II) Complex-Grafted Hollow Hierarchical Metal–Organic Frameworks with Superior Electrochemiluminescence Performance for Sensitive Assay of Thrombin. Anal. Chem. 2021, 93, 6239–6245. [Google Scholar] [CrossRef]
- Zhu, X.; Xing, H.; Xue, Y.; Li, J.; Wang, E.; Dong, S. Atom-Anchoring Strategy with Metal–Organic Frameworks for Highly Efficient Solid-State Electrochemiluminescence. Anal. Chem. 2021, 93, 9628–9633. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, Y.; Li, X.; Dong, X.; Wang, H.; Du, B.; Cao, W.; Wei, Q. Quenching Electrochemiluminescence Immunosensor Based on Resonance Energy Transfer between Ruthenium (II) Complex Incorporated in the UiO-67 Metal–Organic Framework and Gold Nanoparticles for Insulin Detection. ACS Appl. Mater. Interfaces 2018, 10, 22932–22938. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhao, G.; Liu, L.; Li, X.; Wei, Q.; Cao, W. Ultrasensitive competitive method-based electrochemiluminescence immunosensor for diethylstilbestrol detection based on Ru(bpy)32+ as luminophor encapsulated in metal–organic frameworks UiO-67. Biosens. Bioelectron. 2018, 110, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, N.; Wei, D.; Feng, R.; Fan, D.; Hu, L.; Wei, Q.; Ju, H. Double electrochemiluminescence quenching effects of Fe3O4@PDA-CuXO towards self-enhanced Ru(bpy)32+ functionalized MOFs with hollow structure and its application to procalcitonin immunosensing. Biosens. Bioelectron. 2019, 142, 111521. [Google Scholar] [CrossRef]
- Dong, X.; Du, Y.; Zhao, G.; Cao, W.; Fan, D.; Kuang, X.; Wei, Q.; Ju, H. Dual-signal electrochemiluminescence immunosensor for Neuron-specific enolase detection based on “dual-potential” emitter Ru(bpy)32+ functionalized zinc-based metal-organic frameworks. Biosens. Bioelectron. 2021, 192, 113505. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Luo, L.; Cheng, D.; Sun, Y.; Zhang, Y.; Liu, M.; Yao, S. Regulation of the Structure of Zirconium-Based Porphyrinic Metal–Organic Framework as Highly Electrochemiluminescence Sensing Platform for Thrombin. Anal. Chem. 2022, 94, 5707–5714. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.-B.; Xiong, C.-Y.; Liang, W.-B.; Zeng, X.-S.; Xu, H.-L.; Yang, Y.; Yao, L.-Y.; Yuan, R.; Xiao, D.-R. Highly Stable Mesoporous Luminescence-Functionalized MOF with Excellent Electrochemiluminescence Property for Ultrasensitive Immunosensor Construction. ACS Appl. Mater. Interfaces 2018, 10, 15913–15919. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, X.; Yuan, R.; Chai, Y. N-(aminobutyl)-N-(ethylisoluminol) functionalized Fe-based metal-organic frameworks with intrinsic mimic peroxidase activity for sensitive electrochemiluminescence mucin1 determination. Biosens. Bioelectron. 2018, 121, 250–256. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, N.; Li, Y.; Yang, L.; Wei, D.; Yan, T.; Ju, H.; Du, B.; Wei, Q. Cobalt-based metal-organic frameworks as co-reaction accelerator for enhancing electrochemiluminescence behavior of N-(aminobutyl)-N-(ethylisoluminol) and ultrasensitive immunosensing of amyloid-β protein. Sens. Actuators B Chem. 2019, 291, 319–328. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Wang, M.; Li, H.; Saqib, M.; Ge, C.; Zhang, X.; Jin, Y. Enhancing Luminol Electrochemiluminescence by Combined Use of Cobalt-Based Metal Organic Frameworks and Silver Nanoparticles and Its Application in Ultrasensitive Detection of Cardiac Troponin I. Anal. Chem. 2019, 91, 3048–3054. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, Y.; Bao, S.; Wang, N.; Yu, S.; Luo, R.; Ma, J.; Ju, H.; Lei, J. Dual Intrareticular Oxidation of Mixed-Ligand Metal–Organic Frameworks for Stepwise Electrochemiluminescence. J. Am. Chem. Soc. 2021, 143, 3049–3053. [Google Scholar] [CrossRef]
- Wang, J.-M.; Yao, L.-Y.; Huang, W.; Yang, Y.; Liang, W.-B.; Yuan, R.; Xiao, D.-R. Overcoming Aggregation-Induced Quenching by Metal−Organic Framework for Electrochemiluminescence (ECL) Enhancement: Zn-PTC as a New ECL Emitter for Ultrasensitive MicroRNAs Detection. ACS Appl. Mater. Interfaces 2021, 13, 44079–44085. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, A.; Liu, P.; Hou, Y.; Song, L.; Yuan, R.; Fu, Y. Sulfur-functionalized zirconium(IV)-based metal-organic frameworks relieves aggregation-caused quenching effect in efficient electrochemiluminescence sensor. Sens. Actuators B Chem. 2020, 321, 128531. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, J.; Lyu, A.; Wang, M.; Hu, C.; Cui, H. Zn2+-Modified Nonmetal Porphyrin-Based Metal–Organic Frameworks with Improved Electrochemiluminescence for Nanoscale Exosome Detection. ACS Appl. Nano Mater. 2023, 6, 4214–4223. [Google Scholar] [CrossRef]
- Han, Q.; Wang, C.; Liu, P.; Zhang, G.; Song, L.; Fu, Y. Achieving synergistically enhanced dual-mode electrochemiluminescent and electrochemical drug sensors via a multi-effect porphyrin-based metal-organic framework. Sens. Actuators B Chem. 2021, 330, 129388. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Ren, X.; Li, Y.; Liu, L.; Feng, R.; Ma, H.; Wei, Q. Dumbbell Plate-Shaped AIEgen-Based Luminescent MOF with High Quantum Yield as Self-Enhanced ECL Tags: Mechanism Insights and Biosensing Application. Small 2022, 18, 2106567. [Google Scholar] [CrossRef]
- Xiong, X.; Xiong, C.; Gao, Y.; Xiao, Y.; Chen, M.-M.; Wen, W.; Zhang, X.; Wang, S. Tetraphenylethylene-Functionalized Metal–Organic Frameworks with Strong Aggregation-Induced Electrochemiluminescence for Ultrasensitive Analysis through a Multiple Convertible Resonance Energy Transfer System. Anal. Chem. 2022, 94, 7861–7867. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, X.; Yang, C.; Jiang, Y.; Zhen, S.; Huang, C.; Li, Y. Electrochemiluminescence Resonance Energy Transfer System Based on Silver Metal–Organic Frameworks as a Double-Amplified Emitter for Sensitive Detection of miRNA-107. Anal. Chem. 2022, 94, 1178–1186. [Google Scholar] [CrossRef]
- Richter, M.M.; Bard, A.J. Electrogenerated Chemiluminescence. 58. Ligand-Sensitized Electrogenerated Chemiluminescence in Europium Labels. Anal. Chem. 1996, 68, 2641–2650. [Google Scholar] [CrossRef]
- Dong, H.; Liu, S.; Liu, Q.; Li, Y.; Xu, Z.; Li, Y.; Wei, Q. Mixed-Ligand-Regulated Self-Enhanced Luminous Eu-MOF as an ECL Signal Probe for an Oriented Antibody-Decorated Biosensing Platform. Anal. Chem. 2022, 94, 12852–12859. [Google Scholar] [CrossRef]
- Dai, W.; Wang, X.; Chen, G.; Wang, X.; Hu, C.; Zhen, S.; Huang, C.; Li, Y. Facile synthesis of 2D Europium-metal organic frameworks nanosheets for highly efficient electrochemiluminescence in DNA detection. Chem. Eng. J. 2023, 465, 143037. [Google Scholar] [CrossRef]
- Zhao, L.; Song, X.; Ren, X.; Fan, D.; Wei, Q.; Wu, D. Rare Self-Luminous Mixed-Valence Eu-MOF with a Self-Enhanced Characteristic as a Near-Infrared Fluorescent ECL Probe for Nondestructive Immunodetection. Anal. Chem. 2021, 93, 8613–8621. [Google Scholar] [CrossRef]
- Zhao, L.; Song, X.; Ren, X.; Wang, H.; Fan, D.; Wu, D.; Wei, Q. Ultrasensitive near-infrared electrochemiluminescence biosensor derived from Eu-MOF with antenna effect and high efficiency catalysis of specific CoS2 hollow triple shelled nanoboxes for procalcitonin. Biosens. Bioelectron. 2021, 191, 113409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, M.; Song, X.; Liu, X.; Ju, H.; Ai, H.; Wei, Q.; Wu, D. Annihilation luminescent Eu-MOF as a near-infrared electrochemiluminescence probe for trace detection of trenbolone. Chem. Eng. J. 2022, 434, 134691. [Google Scholar] [CrossRef]
- Wang, C.; Han, Q.; Liu, P.; Zhang, G.; Song, L.; Zou, X.; Fu, Y. A Superstable Luminescent Lanthanide Metal Organic Gel Utilized in an Electrochemiluminescence Sensor for Epinephrine Detection with a Narrow Potential Sweep Range. ACS Sens. 2021, 6, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, L.; Wang, C.; Jia, H.; Xue, J.; Wei, Q.; Ju, H. Copper doped terbium metal organic framework as emitter for sensitive electrochemiluminescence detection of CYFRA 21-1. Talanta 2022, 238, 123047. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Ju, H. Copper-Doped Terbium Luminescent Metal Organic Framework as an Emitter and a Co-reaction Promoter for Amplified Electrochemiluminescence Immunoassay. Anal. Chem. 2021, 93, 14878–14884. [Google Scholar] [CrossRef]
- Gao, H.; Wei, X.; Li, M.; Wang, L.; Wei, T.; Dai, Z. Co-Quenching Effect between Lanthanum Metal–Organic Frameworks Luminophore and Crystal Violet for Enhanced Electrochemiluminescence Gene Detection. Small 2021, 17, 2103424. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xiao, S.Y.; Jiang, Z.W.; Zhen, S.J.; Huang, C.Z.; Liu, Q.Q.; Li, Y.F. An ultrathin 2D Yb(III) metal-organic frameworks with strong electrochemiluminescence as a “on-off-on” platform for detection of picric acid and berberine chloride form. Talanta 2021, 234, 122625. [Google Scholar] [CrossRef]
- Li, C.; Liu, L.; Kang, J.; Xiao, Y.; Feng, Y.; Cao, F.-F.; Zhang, H. Pristine MOF and COF materials for advanced batteries. Energy Storage Mater. 2020, 31, 115–134. [Google Scholar] [CrossRef]
- Ren, X.; Liao, G.; Li, Z.; Qiao, H.; Zhang, Y.; Yu, X.; Wang, B.; Tan, H.; Shi, L.; Qi, X.; et al. Two-dimensional MOF and COF nanosheets for next-generation optoelectronic applications. Coord. Chem. Rev. 2021, 435, 213781. [Google Scholar] [CrossRef]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincă, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of MOF and COF Applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001. [Google Scholar] [CrossRef]
- Lv, X.; Li, Y.; Cui, B.; Fang, Y.; Wang, L. Electrochemiluminescent sensor based on an aggregation-induced emission probe for bioanalytical detection. Analyst 2022, 147, 2338–2354. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Yang, Y.; Liang, W.-B.; Yao, L.-Y.; Yuan, R.; Xiao, D.-R. Highly Stable Covalent Organic Framework Nanosheets as a New Generation of Electrochemiluminescence Emitters for Ultrasensitive MicroRNA Detection. Anal. Chem. 2021, 93, 3258–3265. [Google Scholar] [CrossRef]
- Luo, R.; Lv, H.; Liao, Q.; Wang, N.; Yang, J.; Li, Y.; Xi, K.; Wu, X.; Ju, H.; Lei, J. Intrareticular charge transfer regulated electrochemiluminescence of donor–acceptor covalent organic frameworks. Nat. Commun. 2021, 12, 6808. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Cui, W.-R.; Jiang, Q.-Q.; Wu, Q.; Liang, R.-P.; Luo, Q.-X.; Qiu, J.-D. A general design approach toward covalent organic frameworks for highly efficient electrochemiluminescence. Nat. Commun. 2021, 12, 4735. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.-R.; Li, Y.-J.; Jiang, Q.-Q.; Wu, Q.; Luo, Q.-X.; Zhang, L.; Liang, R.-P.; Qiu, J.-D. Covalent Organic Frameworks as Advanced Uranyl Electrochemiluminescence Monitoring Platforms. Anal. Chem. 2021, 93, 16149–16157. [Google Scholar] [CrossRef]
- Li, Y.-J.; Cui, W.-R.; Jiang, Q.-Q.; Liang, R.-P.; Li, X.-J.; Wu, Q.; Luo, Q.-X.; Liu, J.; Qiu, J.-D. Arousing Electrochemiluminescence Out of Non-Electroluminescent Monomers within Covalent Organic Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 47921–47931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-L.; Yao, L.-Y.; Yang, Y.; Liang, W.-B.; Yuan, R.; Xiao, D.-R. Conductive Covalent Organic Frameworks with Conductivity- and Pre-Reduction-Enhanced Electrochemiluminescence for Ultrasensitive Biosensor Construction. Anal. Chem. 2022, 94, 3685–3692. [Google Scholar] [CrossRef]
- Qin, X.; Zhan, Z.; Ding, Z. Progress in electrochemiluminescence biosensors based on organic framework emitters. Curr. Opin. Electrochem. 2023, 39, 101283. [Google Scholar] [CrossRef]
- Hisaki, I.; Xin, C.; Takahashi, K.; Nakamura, T. Designing Hydrogen-Bonded Organic Frameworks (HOFs) with Permanent Porosity. Angew. Chem. Int. Ed. 2019, 58, 11160–11170. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Wang, Y.; Wang, Y.; Luo, R.; Zhu, D.; Zhou, J.; Wu, X.; Ju, H.; Lei, J. Intrareticular electron coupling pathway driven electrochemiluminescence in hydrogen-bonded organic frameworks. J. Mater. Chem. C 2022, 10, 14488–14495. [Google Scholar] [CrossRef]
- Shen, K.-Y.; Zhan, J.; Shen, L.; Xiong, Z.; Zhu, H.-T.; Wang, A.-J.; Yuan, P.-X.; Feng, J.-J. Hydrogen Bond Organic Frameworks as Radical Reactors for Enhancement in ECL Efficiency and Their Ultrasensitive Biosensing. Anal. Chem. 2023, 95, 4735–4743. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-L.; Palacios, R.E.; Fan, F.-R.F.; Bard, A.J.; Barbara, P.F. Electrogenerated Chemiluminescence of Single Conjugated Polymer Nanoparticles. J. Am. Chem. Soc. 2008, 130, 8906–8907. [Google Scholar] [CrossRef]
- Bai, X.; Wang, K.; Chen, L.; Zhou, J.; Wang, J. Semiconducting polymer dots as fluorescent probes for in vitro biosensing. J. Mater. Chem. B 2022, 10, 6248–6262. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hou, W.; Qin, W.; Wu, C. Recent advances in semiconducting polymer dots as optical probes for biosensing. Biomater. Sci. 2021, 9, 328–346. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, N.; Ju, H. Electrochemiluminescence biosensing and bioimaging with nanomaterials as emitters. Sci. China Chem. 2022, 65, 2417–2436. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Luo, Z.; Duan, Y.; Feng, Y. Low-Triggering-Potential Electrochemiluminescence from a Luminol Analogue Functionalized Semiconducting Polymer Dots for Imaging Detection of Blood Glucose. Anal. Chem. 2022, 94, 5615–5623. [Google Scholar] [CrossRef]
- Dai, R.; Wu, F.; Xu, H.; Chi, Y. Anodic, Cathodic, and Annihilation Electrochemiluminescence Emissions from Hydrophilic Conjugated Polymer Dots in Aqueous Medium. ACS Appl. Mater. Interfaces 2015, 7, 15160–15167. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, N.; Ju, H. Highly Efficient Electrochemiluminescence of Cyanovinylene-Contained Polymer Dots in Aqueous Medium and Its Application in Imaging Analysis. Anal. Chem. 2018, 90, 1202–1208. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.; Wang, Y.; Ju, H.; Yan, F. Electrochemiluminescent Imaging for Multi-immunoassay Sensitized by Dual DNA Amplification of Polymer Dot Signal. Anal. Chem. 2018, 90, 7708–7714. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, J.; Liu, D.; He, Y.; Li, Q.; Chen, S.; Yuan, R. A coreactant-free electrochemiluminescence (ECL) biosensor based on in situ generating quencher for the ultrasensitive detection of microRNA. Sens. Actuators B Chem. 2020, 316, 128139. [Google Scholar] [CrossRef]
- Luo, J.-H.; Li, Q.; Chen, S.-H.; Yuan, R. Coreactant-Free Dual Amplified Electrochemiluminescent Biosensor Based on Conjugated Polymer Dots for the Ultrasensitive Detection of MicroRNA. ACS Appl. Mater. Interfaces 2019, 11, 27363–27370. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.; Zhao, J.; Chen, S.; Yuan, R. An ultrasensitive sensing platform for microRNA-155 based on H2O2 quenched hydroxide-dependent ECL emission of PFO Pdots. Biosens. Bioelectron. 2020, 150, 111872. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Xiong, C.; Chai, Y.; Yuan, R. Highly sensitive electrochemiluminescence immunosensor based on ABEI/H2O2 system with PFO dots as enhancer for detection of kidney injury molecule-1. Biosens. Bioelectron. 2018, 116, 16–22. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, F.; Wang, N.; Lei, J.; Ju, H. Ru(bpy)32+ Incorporated Luminescent Polymer Dots: Double-Enhanced Electrochemiluminescence for Detection of Single-Nucleotide Polymorphism. Anal. Chem. 2017, 89, 7659–7666. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, L.; Chen, W.; Ju, H. Potential- and Color-Resolved Electrochemiluminescence of Polymer Dots for Array Imaging of Multiplex MicroRNAs. Anal. Chem. 2021, 93, 5327–5333. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gao, H.; Li, Y.; Li, G.; Chen, W.; Jin, Z.; Lei, J.; Wei, Q.; Ju, H. Dual Intramolecular Electron Transfer for In Situ Coreactant-Embedded Electrochemiluminescence Microimaging of Membrane Protein. Angew. Chem. Int. Ed. 2021, 60, 197–201. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, J.; Li, Q.; Zhou, Y.; Yang, S.; Xu, J.; Hua, D. Improved AIE-Active Probe with High Sensitivity for Accurate Uranyl Ion Monitoring in the Wild Using Portable Electrochemiluminescence System for Environmental Applications. Adv. Funct. Mater. 2020, 30, 2000220. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, H.; Jia, Y.-L.; Pan, J.-B.; Luo, X.-L.; Chen, H.-Y.; Xu, J.-J. Ultrasensitive Nucleic Acid Assay Based on AIE-Active Polymer Dots with Excellent Electrochemiluminescence Stability. Anal. Chem. 2021, 93, 6857–6864. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Ju, H. Electrochemiluminescence nanoemitters for immunoassay of protein biomarkers. Bioelectrochemistry 2023, 149, 108281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, Z.-Y.; Gao, H.; Yu, Y.; Pan, J.-B.; Chen, H.-Y.; Xu, J.-J. An ultrasensitive electrochemiluminescence assay for nucleic acid detection based on carboxyl functionalized polymer dots. J. Electroanal. Chem. 2021, 900, 115743. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, N.; Hu, J.; Pan, J.-B.; Cheng, Y.-X.; Chen, H.-Y.; Xu, J.-J. Molecular Engineering of Polymer Dots for Electrochemiluminescence Emission. ACS Appl. Nano Mater. 2021, 4, 7244–7252. [Google Scholar] [CrossRef]
- Khan, H.; Shah, M.R.; Barek, J.; Malik, M.I. Cancer biomarkers and their biosensors: A comprehensive review. TrAC Trends Anal. Chem. 2023, 158, 116813. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, S.; Tan, L.; Tan, Y.; Wang, Y.; Ye, Z.; Hou, C.; Xu, Y.; Liu, S.; Wang, G. Frontier and hot topics in electrochemiluminescence sensing technology based on CiteSpace bibliometric analysis. Biosens. Bioelectron. 2022, 201, 113932. [Google Scholar] [CrossRef]
- Surinova, S.; Schiess, R.; Hüttenhain, R.; Cerciello, F.; Wollscheid, B.; Aebersold, R. On the Development of Plasma Protein Biomarkers. J. Proteome Res. 2011, 10, 5–16. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Binotti, W.W.; Bayraktutar, B.; Ozmen, M.C.; Cox, S.M.; Hamrah, P. A Review of Imaging Biomarkers of the Ocular Surface. Eye Contact Lens Sci. Clin. Pract. 2020, 46, S84–S105. [Google Scholar] [CrossRef]
- Gao, X.; Liu, X.; Zeng, Y.; Zhang, Q.; Zhang, B.; Zou, G. Spectrum-Resolved Electrochemiluminescence to Multiplex the Immunoassay and DNA Probe Assay. Anal. Chem. 2022, 94, 15801–15808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.-J.; Wang, X.; Zhang, Y.-H.; Qu, J.; Ding, S.-N. Band-Edge Effect-Induced Electrochemiluminescence Signal Amplification Based on Inverse Opal Photonic Crystals for Ultrasensitive Detection of Carcinoembryonic Antigen. Anal. Chem. 2022, 94, 9919–9926. [Google Scholar] [CrossRef]
- Huang, Y.; Lei, J.; Cheng, Y.; Ju, H. Ratiometric electrochemiluminescent strategy regulated by electrocatalysis of palladium nanocluster for immunosensing. Biosens. Bioelectron. 2016, 77, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-S.; Yuan, D.-J.; Xu, J.-J.; Chen, H.-Y. Electrochemiluminescence on bipolar electrodes for visual bioanalysis. Chem. Sci. 2013, 4, 1182–1188. [Google Scholar] [CrossRef]
- Li, X.; Qin, X.; Tian, Z.; Wang, K.; Xia, X.; Wu, Y.; Liu, S. Gold Nanowires Array-Based Closed Bipolar Nanoelectrode System for Electrochemiluminescence Detection of α-Fetoprotein on Cell Surface. Anal. Chem. 2022, 94, 7350–7357. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, Q.; Kang, Q.; Zhang, B.; Shen, D.; Zou, G. Near-Infrared Electrochemiluminescence Immunoassay with Biocompatible Au Nanoclusters as Tags. Anal. Chem. 2020, 92, 7581–7587. [Google Scholar] [CrossRef]
- Wang, Y.; Kan, X. Sensitive and selective “signal-off” electrochemiluminescence sensing of prostate-specific antigen based on an aptamer and molecularly imprinted polymer. Analyst 2021, 146, 7693–7701. [Google Scholar] [CrossRef]
- Qin, D.; Meng, S.; Wu, Y.; Mo, G.; Deng, B. Aggregation-induced electrochemiluminescence resonance energy transfer with dual quenchers for the sensitive detection of prostate-specific antigen. Sens. Actuators B Chem. 2022, 367, 132176. [Google Scholar] [CrossRef]
- Zhao, L.; Song, X.; Fan, D.; Liu, X.; Wang, H.; Wei, Q.; Wu, D. Highly Efficient Signal On/Off Electrochemiluminescence Gel Aptasensor Based on a Controlled Release Strategy for the Sensitive Detection of Prostate Specific Antigen. Anal. Chem. 2023, 95, 5695–5701. [Google Scholar] [CrossRef]
- Fu, L.; Gao, X.; Dong, S.; Jia, J.; Xu, Y.; Wang, D.; Zou, G. Coreactant-Free and Direct Electrochemiluminescence from Dual-Stabilizer-Capped InP/ZnS Nanocrystals: A New Route Involving n-Type Luminophore. Anal. Chem. 2022, 94, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, T.; Du, Y.; Zhang, N.; Feng, R.; Ma, H.; Wei, Q. PEGylation Improved Electrochemiluminescence Supramolecular Assembly of Iridium(III) Complexes in Apoferritin for Immunoassays Using 2D/2D MXene/TiO2 Hybrids as Signal Amplifiers. Anal. Chem. 2021, 93, 16906–16914. [Google Scholar] [CrossRef]
- Yang, L.; Du, Y.; Fan, D.; Zhang, Y.; Kuang, X.; Sun, X.; Wei, Q. Facile Encapsulation of Iridium(III) Complexes in Apoferritin Nanocages as Promising Electrochemiluminescence Nanodots for Immunoassays. Anal. Chem. 2021, 93, 11329–11336. [Google Scholar] [CrossRef]
- Han, Y.; Jia, Y.; Du, Y.; Li, Y.; Ren, X.; Ma, H.; Wu, D.; Kuang, X.; Fan, D.; Wei, Q. Controlled Growth of MoS2 on Dendritic Ferric Oxide to Enhance Electrochemiluminescence of Nitrogen-Doped Carbon Quantum Dots for Sensitive Immunoassay. Anal. Chem. 2023, 95, 6655–6663. [Google Scholar] [CrossRef]
- Du, Y.; Xue, J.; Sun, X.; Wu, D.; Liu, X.; Ju, H.; Yang, L.; Wei, Q. Oxygen Vacancy-Enhanced Electrochemiluminescence Sensing Strategy Using Luminol Thermally Encapsulated in Apoferritin as a Transducer for Biomarker Immunoassay. Anal. Chem. 2020, 92, 8472–8479. [Google Scholar] [CrossRef]
- Xue, J.; Jia, Y.; Yang, L.; Feng, J.; Wu, D.; Ren, X.; Du, Y.; Ju, H.; Wei, Q. Etching Triangular Silver Nanoparticles by Self-generated Hydrogen Peroxide to Initiate the Response of an Electrochemiluminescence Sensing Platform. Anal. Chem. 2020, 92, 14203–14209. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, X.; Wei, D.; Ju, H.; Du, Y.; Ma, H.; Wei, Q. Aggregation-Induced Electrochemiluminescence Bioconjugates of Apoferritin-Encapsulated Iridium(III) Complexes for Biosensing Application. Anal. Chem. 2021, 93, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, Y.; Wang, H.; Ma, H.; Wu, D.; Ren, X.; Wei, Q.; Xu, J.-J. Self-Supply of H2O2 and O2 by Hydrolyzing CaO2 to Enhance the Electrochemiluminescence of Luminol Based on a Closed Bipolar Electrode. Anal. Chem. 2020, 92, 12693–12699. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Qian, X.; Mi, X.; Tu, Y. A novel electrochemiluminescent immunosensor for the detection of NT-proBNP based on a Au/ZIF-67 nanocomposite. J. Electroanal. Chem. 2022, 912, 116260. [Google Scholar] [CrossRef]
- Yang, L.; Jia, Y.; Wu, D.; Zhang, Y.; Ju, H.; Du, Y.; Ma, H.; Wei, Q. Synthesis and Application of CeO2/SnS2 Heterostructures as a Highly Efficient Coreaction Accelerator in the Luminol–Dissolved O2 System for Ultrasensitive Biomarkers Immunoassay. Anal. Chem. 2019, 91, 14066–14073. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, L.; Feng, R.; Ma, H.; Fan, D.; Yan, T.; Feng, R.; Du, B.; Wei, Q. MnCO3 as a New Electrochemiluminescence Emitter for Ultrasensitive Bioanalysis of β-Amyloid1–42 Oligomers Based on Site-Directed Immobilization of Antibody. ACS Appl. Mater. Interfaces 2019, 11, 7157–7163. [Google Scholar] [CrossRef]
- Tan, R.; Wang, Y.; Mi, X.; Li, H.; Tu, Y. A dual-screening electrochemiluminescent aptasensor based on a mesoporous silica nano-sieve for specific detection of amyloid-β monomer. Sens. Actuators B Chem. 2022, 352, 131065. [Google Scholar] [CrossRef]
- Ali, A.; Zhao, J.; Khan, M.S.; Wang, H.; Ren, X.; Hu, L.; Manzoor, R.; Wu, D.; Wei, Q. Electrochemiluminescence detection for β-amyloid1-42 oligomers using silver nanoparticle decorated CuS@CoS2 double shelled nanoboxes as dual-quencher. Sens. Actuators B Chem. 2021, 329, 129155. [Google Scholar] [CrossRef]
- Qin, D.; Meng, S.; Wu, Y.; Mo, G.; Jiang, X.; Deng, B. Design of a Dual-Wavelength Ratiometric Electrochemiluminescence Immunosensor for Sensitive Detection of Amyloid-β Protein in Human Serum. ACS Sustain. Chem. Eng. 2021, 9, 7541–7549. [Google Scholar] [CrossRef]
- Xu, M.-R.; Dai, R.-F.; Wei, Q.-Q.; Wang, J.; Feng, Y.-Y.; Hu, Y. Urinary AD7c-NTP Evaluates Cognition Impairment and Differentially Diagnoses AD and MCI. Am. J. Alzheimers Dis. Dement. 2022, 37, 153331752211152. [Google Scholar] [CrossRef]
- Liang, Y.; Xue, K.; Shi, Y.; Zhan, T.; Lai, W.; Zhang, C. Dry Chemistry-Based Bipolar Electrochemiluminescence Immunoassay Device for Point-of-Care Testing of Alzheimer-Associated Neuronal Thread Protein. Anal. Chem. 2023, 95, 3434–3441. [Google Scholar] [CrossRef] [PubMed]
- Domenico, T.; Rita, A.; Giacomo, S.; Diego, A.; Thelma, P.; Mariana, G.; Giampaolo, N.; Francesco, N.; Maria, G.; Francesco, F.; et al. Salivary biomarkers for diagnosis of acute myocardial infarction: A systematic review. Int. J. Cardiol. 2023, 371, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Gumanova, N.G.; Bogdanova, N.L.; Metelskaya, V.A.; Tarasov, V.I.; Kots, A.Y.; Kutsenko, V.A.; Kontsevaya, A.V.; Drapkina, O.M. Serum biomarkers, including nitric oxide metabolites (NOx), for prognosis of cardiovascular death and acute myocardial infarction in an ESSE-RF case–control cohort with 6.5-year follow up. Sci. Rep. 2022, 12, 18177. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, Y.; Huang, X.; Hang, J.; Guo, W.; Dai, Z. Multicolor Iridium(III) Complexes with Host–Guest Recognition Motifs for Enhanced Electrochemiluminescence and Modular Labeling. Anal. Chem. 2023, 95, 4543–4549. [Google Scholar] [CrossRef]
- Hong, D.; Kim, K.; Jo, E.-J.; Kim, M.-G. Electrochemiluminescence-Incorporated Lateral Flow Immunosensors Using Ru(bpy)32+-Labeled Gold Nanoparticles for the Full-Range Detection of Physiological C-Reactive Protein Levels. Anal. Chem. 2021, 93, 7925–7932. [Google Scholar] [CrossRef]
- Guo, X.-M.; Zhao, M.-L.; Liang, W.-B.; Yang, X.; Yuan, R.; Zhuo, Y. Programmable Y-Shaped Probes with Proximity Bivalent Recognition for Rapid Electrochemiluminescence Response of Acute Myocardial Infarction. ACS Sens. 2022, 7, 3208–3215. [Google Scholar] [CrossRef]
- Ji, Y.; He, S.; Chen, Y.; Zhang, P.; Sun, J.; Li, Y.; Kuang, K.; Jia, N. A sensitive dual-signal electrochemiluminescence immunosensor based on Ru(bpy)32+ @HKUST-1 and Ce2Sn2O7 for detecting the heart failure biomarker NT-proBNP. J. Mater. Chem. B 2023, 11, 2754–2761. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, Y.; Li, X.; Ren, X.; Fan, D.; Wang, H.; Wei, Q.; Ju, H. Electrochemiluminescence immunosensor based on ferrocene functionalized ZIF-8 quenching the electrochemiluminescence of Ru(bpy)32+-doped silica nanoparticles embodied N-butyldiethanolamine. Sens. Actuators B Chem. 2021, 329, 129101. [Google Scholar] [CrossRef]
- Song, C.; Li, X.; Hu, L.; Shi, T.; Wu, D.; Ma, H.; Zhang, Y.; Fan, D.; Wei, Q.; Ju, H. Quench-Type Electrochemiluminescence Immunosensor Based on Resonance Energy Transfer from Carbon Nanotubes and Au-Nanoparticles-Enhanced g-C3N4 to CuO@Polydopamine for Procalcitonin Detection. ACS Appl. Mater. Interfaces 2020, 12, 8006–8015. [Google Scholar] [CrossRef]
- Wang, N.; Yang, J.; Luo, Z.; Qin, D.; Wu, Y.; Deng, B. A dual-emitting immunosensor based on manganese dioxide nanoflowers and zinc sulfide quantum dots with enhanced electrochemiluminescence performance for the ultrasensitive detection of procalcitonin. Analyst 2023, 148, 2122–2132. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, L.; Zhang, N.; Liu, L.; Ren, X.; Ma, H.; Kuang, X.; Li, Y.; Luo, C.; Wei, Q. Ultrasensitive Electrochemiluminescence Biosensor with Silver Nanoclusters as a Novel Signal Probe and α-Fe2O3–Pt as an Efficient Co-reaction Accelerator for Procalcitonin Immunoassay. Anal. Chem. 2023, 95, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jiang, D.; Zhu, J.-J. High-resolution imaging of catalytic activity of a single graphene sheet using electrochemiluminescence microscopy. Chem. Sci. 2021, 12, 4794–4799. [Google Scholar] [CrossRef]
- Knezevic, S.; Bouffier, L.; Liu, B.; Jiang, D.; Sojic, N. Electrochemiluminescence microscopy: From single objects to living cells. Curr. Opin. Electrochem. 2022, 35, 101096. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, J.; Zhao, P.; Zhang, J.; Zheng, M.; Feng, J. Imaging of Single Bacteria with Electrochemiluminescence Microscopy. J. Am. Chem. Soc. 2023, 145, 8947–8953. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Yao, M.; Han, W.; Zhang, S. Cathodic Electrochemiluminesence Microscopy for Imaging of Single Carbon Nanotube and Nucleolin at Single Tumor Cell. Anal. Chem. 2023, 95, 570–574. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, X.; Wang, S.; Li, B.; Liu, B. Nanoconfinement-Enhanced Electrochemiluminescence for in Situ Imaging of Single Biomolecules. ACS Nano 2023, 17, 3809–3817. [Google Scholar] [CrossRef]
- Liu, G.; Jin, B.-K.; Ma, C.; Chen, Z.; Zhu, J.-J. Potential-Resolved Electrochemiluminescence Nanoprobes for Visual Apoptosis Evaluation at Single-Cell Level. Anal. Chem. 2019, 91, 6363–6370. [Google Scholar] [CrossRef]
- Chen, Y.; Gou, X.; Ma, C.; Jiang, D.; Zhu, J.-J. A Synergistic Coreactant for Single-Cell Electrochemiluminescence Imaging: Guanine-Rich ssDNA-Loaded High-Index Faceted Gold Nanoflowers. Anal. Chem. 2021, 93, 7682–7689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, R.; Jiang, D.; Chen, H.-Y. Electrochemiluminescence-Based Capacitance Microscopy for Label-Free Imaging of Antigens on the Cellular Plasma Membrane. J. Am. Chem. Soc. 2019, 141, 10294–10299. [Google Scholar] [CrossRef]
- Chen, M.; Xu, C.; Zhao, W.; Chen, H.; Xu, J. Single Cell Imaging of Electrochemiluminescence-Driven Photodynamic Therapy. Angew. Chem. Int. Ed. 2022, 61, e2021174. [Google Scholar] [CrossRef]
- Ding, H.; Guo, W.; Su, B. Imaging Cell-Matrix Adhesions and Collective Migration of Living Cells by Electrochemiluminescence Microscopy. Angew. Chem. Int. Ed. 2020, 59, 449–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, L.; Ding, H.; Zhou, P.; Xi, L.; Su, B. Surface-Sensitive Imaging Analysis of Cell–Microenvironment Interactions by Electrochemiluminescence Microscopy. Anal. Chem. 2022, 94, 10885–10892. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Li, J. Recent Advances in Electrochemiluminescence Emitters for Biosensing and Imaging of Protein Biomarkers. Chemosensors 2023, 11, 432. https://doi.org/10.3390/chemosensors11080432
Yang L, Li J. Recent Advances in Electrochemiluminescence Emitters for Biosensing and Imaging of Protein Biomarkers. Chemosensors. 2023; 11(8):432. https://doi.org/10.3390/chemosensors11080432
Chicago/Turabian StyleYang, Lei, and Jinghong Li. 2023. "Recent Advances in Electrochemiluminescence Emitters for Biosensing and Imaging of Protein Biomarkers" Chemosensors 11, no. 8: 432. https://doi.org/10.3390/chemosensors11080432