Theoretical Study of 3d VIII Atom-Decorated γ-Graphyne for Adsorbing and Detecting Heptafluoroisobutyronitrile
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Structure of C4F7N, Pristine and 3d VIII Atom-Decorated γ-GY
3.2. Adsorption of C4F7N on Different Single 3d VIII TM Atom-Decorated γ-GY
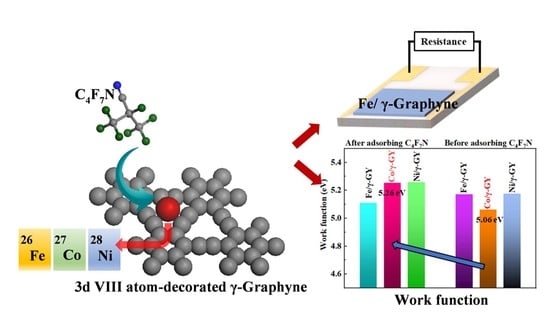
3.3. Electronic Properties of Single 3d VIII TM Atom-Decorated γ-GY before and after Adsorbing C4F7N
3.4. Sensing Properties of Single 3d VIII TM Atom-Decorated γ-GY to Detect C4F7N
3.5. Thermodynamic Analysis of Adsorption Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beroual, A.; Haddad, A. Recent advances in the quest for a new insulation gas with a low impact on the environment to replace sulfur hexafluoride (SF6) gas in high-voltage power network applications. Energies 2017, 10, 1216. [Google Scholar] [CrossRef] [Green Version]
- Osipov, A.A.; Iankevich, G.A.; Speshilova, A.B.; Osipov, A.A.; Endiiarova, E.V.; Berezenko, V.I.; Tyurikova, I.A.; Tyurikov, K.S.; Alexandrov, S.E. High-temperature etching of sic in SF6/O2 inductively coupled plasma. Sci. Rep. 2020, 10, 19977. [Google Scholar] [CrossRef] [PubMed]
- Sulbaek Andersen, M.P.; Kyte, M.; Andersen, S.T.; Nielsen, C.J.; Nielsen, O.J. Atmospheric chemistry of (cf3)2cf–c≡n: A replacement compound for the most potent industrial greenhouse gas, SF6. Environ. Sci. Technol. 2017, 51, 1321–1329. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Murphy, A.B. SF6-alternative gases for application in gas-insulated switchgear. J. Phys. D Appl. Phys. 2018, 51, 153001. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Xiao, S.; Chen, Q.; Tang, J.; Chen, D.; Wang, D. Decomposition properties of C4F7N/N2 gas mixture: An environmentally friendly gas to replace SF6. Ind. Eng. Chem. Res. 2018, 57, 5173–5182. [Google Scholar] [CrossRef]
- Kieffel, Y.; Irwin, T.; Ponchon, P.; Owens, J. Green gas to replace SF6 in electrical grids. IEEE Power Energy Mag. 2016, 14, 32–39. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, D.; Tang, J.; Li, Y. Adsorption behavior of γ-Al2O3 toward heptafluoroisobutyronitrile and its decompositions: Theoretical and experimental insights. IEEE Access 2020, 8, 36741–36748. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, B.; Yang, T.; Deng, Y.; Li, X.; Murphy, A.B. Thermal decomposition characteristics and kinetic analysis of C4F7N/CO2 gas mixture. J. Phys. D Appl. Phys. 2020, 53, 055502. [Google Scholar] [CrossRef]
- Material Toxicity Summary Sheet, 3M™ Novec™ 4710 Insulating Gas; 3M Company: St. Paul, MN, USA, 2019.
- Li, Y.; Zhang, X.; Zhang, J.; Xiao, S.; Xie, B.; Chen, D.; Gao, Y.; Tang, J. Assessment on the toxicity and application risk of C4F7N: A new SF6 alternative gas. J. Hazard. Mater. 2019, 368, 653–660. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, K.; Wu, C.; Zhao, J.; Xie, Y. Surface chemical-modification for engineering the intrinsic physical properties of inorganic two-dimensional nanomaterials. Chem. Soc. Rev. 2015, 44, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Wang, K.; Zhu, H. Recent developments in graphene-based membranes: Structure, mass-transport mechanism and potential applications. Adv. Mater. 2016, 28, 2287–2310. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Liu, X.; Yu, W. Research progress of gas sensor based on graphene and its derivatives: A review. Appl. Sci. 2018, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Wang, J.; Wang, Y. Strategies for the performance enhancement of graphene-based gas sensors: A review. Talanta 2021, 235, 122745. [Google Scholar] [CrossRef]
- Kharche, N.; Nayak, S.K. Quasiparticle band gap engineering of graphene and graphone on hexagonal boron nitride substrate. Nano Lett. 2011, 11, 5274–5278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Lim, J.H.; Lv, Y.; Li, N.; Kang, B.; Lee, J.Y. Graphynes and graphdiynes for energy storage and catalytic utilization: Theoretical insights into recent advances. Chem. Rev. 2023, 123, 4795–4854. [Google Scholar] [CrossRef] [PubMed]
- Narita, N.; Nagai, S.; Suzuki, S.; Nakao, K. Optimized geometries and electronic structures of graphyne and its family. Phys. Rev. B 1998, 58, 11009–11014. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Chen, Y.; Wu, L.; Yang, C.; Cui, X. Synthesis of γ-graphyne by mechanochemistry and its electronic structure. Carbon 2018, 136, 248–254. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, C.; Pan, Q.; Jin, Y.; Lyu, R.; Martinez, V.; Huang, S.; Wu, J.; Wayment, L.J.; Clark, N.A.; et al. Synthesis of γ-graphyne using dynamic covalent chemistry. Nat. Synth. 2022, 1, 449–454. [Google Scholar] [CrossRef]
- Kim, S.; Ruiz Puigdollers, A.; Gamallo, P.; Viñes, F.; Lee, J.Y. Functionalization of γ-graphyne by transition metal adatoms. Carbon 2017, 120, 63–70. [Google Scholar] [CrossRef]
- He, J.; Zhou, P.; Jiao, N.; Ma, S.Y.; Zhang, K.W.; Wang, R.Z.; Sun, L.Z. Magnetic exchange coupling and anisotropy of 3d transition metal nanowires on graphyne. Sci. Rep. 2014, 4, 4014. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Zhang, X.; Tang, J.; Cui, H.; Li, Y.; Zhang, G.; Yang, J. Density functional theory study of small Ag cluster adsorbed on graphyne. Appl. Surf. Sci. 2019, 465, 93–102. [Google Scholar] [CrossRef]
- Lu, Z.; Lv, P.; Ma, D.; Yang, X.; Li, S.; Yang, Z. Detection of gas molecules on single mn adatom adsorbed graphyne: A dft-d study. J. Phys. D Appl. Phys. 2018, 51, 065109. [Google Scholar] [CrossRef]
- Ma, D.W.; Li, T.; Wang, Q.; Yang, G.; He, C.; Ma, B.; Lu, Z. Graphyne as a promising substrate for the noble-metal single-atom catalysts. Carbon 2015, 95, 756–765. [Google Scholar] [CrossRef]
- He, C.; Wang, R.; Xiang, D.; Li, X.; Fu, L.; Jian, Z.; Huo, J.; Li, S. Charge-regulated CO2 capture capacity of metal atom embedded graphyne: A first-principles study. Appl. Surf. Sci. 2020, 509, 145392. [Google Scholar] [CrossRef]
- Li, S.-L.; Li, Q.; Chen, Y.; Zhao, Y.; Gan, L.-Y. Transition metal embedded graphynes as advanced bifunctional single atom catalysts for oxygen reduction and evolution reactions. Appl. Surf. Sci. 2022, 605, 154828. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Y.; Tan, Y.; Liu, S.; Cheng, Z.; Shen, Z. Graphyne doped with transition-metal single atoms as effective bifunctional electrocatalysts for water splitting. Appl. Surf. Sci. 2019, 492, 8–15. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chem. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Chen, D.; Xiao, S.; Tian, S.; Tang, J.; Wang, D. Dissociative adsorption of environment-friendly insulating medium C3F7CN on Cu(111) and Al(111) surface: A theoretical evaluation. Appl. Surf. Sci. 2018, 434, 549–560. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, A.; Wang, X.; Rong, M. Theoretical study of the decomposition mechanism of C4F7N. J. Phys. D Appl. Phys. 2019, 52, 245203. [Google Scholar] [CrossRef]
- Ruiz-Puigdollers, A.; Gamallo, P. Dft study of the role of n- and b-doping on structural, elastic and electronic properties of α-, β- and γ-graphyne. Carbon 2017, 114, 301–310. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Z.; Wu, W.; Liu, Y.; Zhou, Z. Adsorption of nox (x = 1, 2) gas molecule on pristine and b atom embedded γ-graphyne based on first-principles study. Appl. Surf. Sci. 2018, 455, 484–491. [Google Scholar] [CrossRef]
- Philipsen, P.H.T.; Baerends, E.J. Cohesive energy of 3d transition metals: Density functional theory atomic and bulk calculations. Phys. Rev. B 1996, 54, 5326–5333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendavid, L.I.; Carter, E.A. CO2 adsorption on Cu2O(111): A DFT+U and DFT-D study. J. Phys. Chem. C 2013, 117, 26048–26059. [Google Scholar] [CrossRef]
- Kou, L.; Frauenheim, T.; Chen, C. Phosphorene as a superior gas sensor: Selective adsorption and distinct I–V response. J. Phys. Chem. Lett. 2014, 5, 2675–2681. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Chen, X.; Weng, K.; Arramel; Jiang, J.; Ong, W.-J.; Zhang, P.; Zhao, X.; Li, N. Highly sensitive and selective gas sensor using heteroatom doping graphdiyne: A DFT study. Adv. Electron. Mater. 2021, 7, 2001244. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, Z.; Cui, H. Pristine and cu decorated hexagonal inn monolayer, a promising candidate to detect and scavenge sf6 decompositions based on first-principle study. J. Hazard. Mater. 2019, 363, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhai, Y.; Liang, Q.; Wu, W. Promoting sensitivity and selectivity of NO2 gas sensor based on metal (Pt, Re, Ta)-doped monolayer WSe2: A DFT study. Chem. Phys. Lett. 2020, 755, 137737. [Google Scholar] [CrossRef]
- Flietner, B.; Doll, T.; Lechner, J.; Leu, M.; Eisele, I. Reliable hybrid GasFETs for work-function measurements with arbitrary materials. Sens. Actuators B 1994, 22, 109–113. [Google Scholar] [CrossRef]
- Eisele, I.; Doll, T.; Burgmair, M. Low power gas detection with FET sensors. Sens. Actuators B 2001, 78, 19–25. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, L.; Xu, S.; Shi, M.; Wu, W.; Zhang, K. NH3, PH3 and AsH3 adsorption on alkaline earth metal (Be-Sr) doped graphenes: Insights from DFT calculations. Appl. Surf. Sci. 2021, 537, 147542. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K.; Qi, P.; Dai, H. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Chen, Y.-B.; Zhou, K.-G.; Liu, C.-H.; Zeng, J.; Zhang, H.-L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Li, L.; Huang, H.; Xu, S.; Yan, G.; Zhao, M.; Ding, Z. Adsorption characteristics of acid gases (NO, NO2, SO2 and SO3) on different single-atom nickel adsorbent: A first-principles study. Appl. Surf. Sci. 2020, 527, 146939. [Google Scholar] [CrossRef]








| Element | Binding Energy (eV) | Distance (Å) | Hirshfeld Charge (e) | Magnetic Moment of TM Atom (μB) |
|---|---|---|---|---|
| Fe/γ-GY | −4.57 | 1.97 | +0.16 | +2.13 |
| Co/γ-GY | −5.14 | 1.94 | +0.07 | +1.03 |
| Ni/γ-GY | −5.23 | 1.95 | +0.03 | 0.00 |
| Adsorption Structure | Eads (eV) | Eads with Out DFT-D2 (eV) | QT (e) | Magnetic Moment of TM Atom after Adsorption (μB) |
|---|---|---|---|---|
| Fe/γ-GY | −1.02 | −0.77 | +0.01 | 0 |
| Co/γ-GY | −0.66 | −0.35 | +0.06 | +0.75 |
| Ni/γ-GY | −0.28 | −0.01 | +0.04 | 0 |
| Pristine γ-GY | −0.27 | −0.01 | +0.04 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Zhao, R.; Chen, D.; Miao, Q.; Liu, K.; Xiao, B. Theoretical Study of 3d VIII Atom-Decorated γ-Graphyne for Adsorbing and Detecting Heptafluoroisobutyronitrile. Chemosensors 2023, 11, 411. https://doi.org/10.3390/chemosensors11070411
Zheng Z, Zhao R, Chen D, Miao Q, Liu K, Xiao B. Theoretical Study of 3d VIII Atom-Decorated γ-Graphyne for Adsorbing and Detecting Heptafluoroisobutyronitrile. Chemosensors. 2023; 11(7):411. https://doi.org/10.3390/chemosensors11070411
Chicago/Turabian StyleZheng, Ziang, Renchu Zhao, Dachang Chen, Qing Miao, Ke Liu, and Beibei Xiao. 2023. "Theoretical Study of 3d VIII Atom-Decorated γ-Graphyne for Adsorbing and Detecting Heptafluoroisobutyronitrile" Chemosensors 11, no. 7: 411. https://doi.org/10.3390/chemosensors11070411