Electrochemical Sensor for Food Monitoring Using Metal-Organic Framework Materials
Abstract
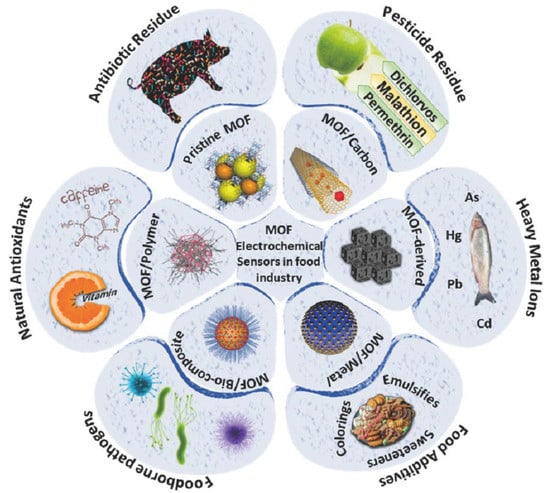
:1. Introduction
2. Application of MOF-Based Materials for Electrochemical Food Monitoring
2.1. Heavy Metal Ions
2.2. Food Additives
2.3. Foodborne Pathogens
2.4. Pesticide Residues
2.5. Hydrogen Peroxide
2.6. Antibiotic Residue
2.7. Antioxidant Compounds
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, C. Analytical methods for monitoring contaminants in food—An industrial perspective. J. Chromatogr. A 2009, 1216, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Fu, Y.; Guo, Y.; Zhang, Q.; Zhang, Q.; Yang, H.; Li, Y. A water-stable luminescent metal–organic framework for effective detection of aflatoxin B1 in walnut and almond beverages. RSC Adv. 2019, 9, 620–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pechprasarn, S.; Ittipornnuson, K.; Jungpanich, T.; Pensupa, N.; Albutt, N. Surface plasmon biosensor platform for food industry. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2019; pp. 103–108. [Google Scholar]
- Xing, G.; Sun, X.; Li, N.; Li, X.; Wu, T.; Wang, F. New Advances in Lateral Flow Immunoassay (LFI) Technology for Food Safety Detection. Molecules 2022, 27, 6596. [Google Scholar] [CrossRef] [PubMed]
- Alikord, M.; Mohammadi, A.; Kamankesh, M.; Shariatifar, N. Food safety and quality assessment: Comprehensive review and recent trends in the applications of ion mobility spectrometry (IMS). Crit. Rev. Food Sci. Nutr. 2022, 62, 4833–4866. [Google Scholar] [CrossRef] [PubMed]
- Špánik, I.; Machyňáková, A. Recent applications of gas chromatography with high-resolution mass spectrometry. J. Sep. Sci. 2018, 41, 163–179. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, B.; Shen, C.; Cheong, L.-Z. Direct, selective and ultrasensitive electrochemical biosensing of methyl parathion in vegetables using Burkholderia cepacia lipase@ MOF nanofibers-based biosensor. Talanta 2019, 197, 356–362. [Google Scholar] [CrossRef]
- Zeng, L.; Peng, L.; Wu, D.; Yang, B. Electrochemical sensors for food safety. Nutrition in Health and Disease–Our Challenges Now and Forthcoming Times; BoD—Books on Demand: Norderstedt, Germany, 2018. [Google Scholar]
- Curulli, A. Electrochemical biosensors in food safety: Challenges and perspectives. Molecules 2021, 26, 2940. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Adhikari, B.; Chen, A. Nanomaterial based electrochemical sensors for the safety and quality control of food and beverages. Analyst 2018, 143, 4537–4554. [Google Scholar] [CrossRef]
- Lv, M.; Liu, Y.; Geng, J.; Kou, X.; Xin, Z.; Yang, D. Engineering nanomaterials-based biosensors for food safety detection. Biosens. Bioelectron. 2018, 106, 122–128. [Google Scholar] [CrossRef]
- Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.; Sun, Y.; Chu, J.; Zhang, X.; Deng, H. Multivariate metal–organic frameworks for dialing-in the binding and programming the release of drug molecules. J. Am. Chem. Soc. 2017, 139, 14209–14216. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Nagar, B.; Benages-Vilau, R.; Gomez-Romero, P.; Kazemi, S.H. MOF-derived conformal cobalt oxide/C composite material as high-performance electrode in hybrid supercapacitors. Electrochim. Acta 2021, 389, 138657. [Google Scholar] [CrossRef]
- Kazemi, S.H.; Hosseinzadeh, B.; Kazemi, H.; Kiani, M.A.; Hajati, S. Facile synthesis of mixed metal–organic frameworks: Electrode materials for supercapacitors with excellent areal capacitance and operational stability. ACS Appl. Mater. Interfaces 2018, 10, 23063–23073. [Google Scholar] [CrossRef] [PubMed]
- Hira, S.A.; Nallal, M.; Rajendran, K.; Song, S.; Park, S.; Lee, J.-M.; Joo, S.H.; Park, K.H. Ultrasensitive detection of hydrogen peroxide and dopamine using copolymer-grafted metal-organic framework based electrochemical sensor. Anal. Chim. Acta 2020, 1118, 26–35. [Google Scholar] [CrossRef] [PubMed]
- eun Kim, S.; Muthurasu, A. Metal-organic framework–assisted bimetallic Ni@ Cu microsphere for enzyme-free electrochemical sensing of glucose. J. Electroanal. Chem. 2020, 873, 114356. [Google Scholar] [CrossRef]
- Xiang, X.; Pan, F.; Li, Y. Flower-like bismuth metal-organic frameworks grown on carbon paper as a free-standing electrode for efficient electrochemical sensing of Cd2+ and Pb2+ in water. Eng. Sci. 2018, 3, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.; Wang, Q.; Qiao, J.; Xu, Y.; Li, G. In situ synthesis of sandwich MOFs on reduced graphene oxide for electrochemical sensing of dihydroxybenzene isomers. Analyst 2019, 144, 2120–2129. [Google Scholar] [CrossRef]
- Cheng, W.; Tang, X.; Zhang, Y.; Wu, D.; Yang, W. Applications of metal-organic framework (MOF)-based sensors for food safety: Enhancing mechanisms and recent advances. Trends Food Sci. Technol. 2021, 112, 268–282. [Google Scholar] [CrossRef]
- Zhang, Z.; Lou, Y.; Guo, C.; Jia, Q.; Song, Y.; Tian, J.-Y.; Zhang, S.; Wang, M.; He, L.; Du, M. Metal–organic frameworks (MOFs) based chemosensors/biosensors for analysis of food contaminants. Trends Food Sci. Technol. 2021, 118, 569–588. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.S.; Jiao, T.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based sensors for the detection of food contaminants. TrAC Trends Anal. Chem. 2022, 154, 116642. [Google Scholar] [CrossRef]
- Hitabatuma, A.; Wang, P.; Su, X.; Ma, M. Metal-Organic Frameworks-Based Sensors for Food Safety. Foods 2022, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Ye, Y.; Qiu, Y.; Liu, J.; Huang, L.; Liang, B.; Chen, B. A Fluorescent Metal–Organic Framework for Food Real-Time Visual Monitoring. Adv. Mater. 2021, 33, 2008020. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Li, G.; Xu, F.; Zhang, Z. Metal–organic frameworks: Opportunities and challenges for surface-enhanced Raman scattering—A review. J. Mater. Chem. C 2020, 8, 2952–2963. [Google Scholar] [CrossRef]
- Lyu, F.; Zhang, Y.; Zare, R.N.; Ge, J.; Liu, Z. One-pot synthesis of protein-embedded metal–organic frameworks with enhanced biological activities. Nano Lett. 2014, 14, 5761–5765. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lou, J.; Yang, L.; Liu, M.; Xia, N.; Liu, L. Design and Application of Electrochemical Sensors with Metal–Organic Frameworks as the Electrode Materials or Signal Tags. Nanomaterials 2022, 12, 3248. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Coord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Mao, Y.; Li, J.; Cao, W.; Ying, Y.; Hu, P.; Liu, Y.; Sun, L.; Wang, H.; Jin, C.; Peng, X. General incorporation of diverse components inside metal-organic framework thin films at room temperature. Nat. Commun. 2014, 5, 5532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Mu, C.; Yan, B.; Qin, X.; Shen, C.; Xue, H.; Pang, H. Nanoparticle/MOF composites: Preparations and applications. Mater. Horiz. 2017, 4, 557–569. [Google Scholar] [CrossRef]
- Kalaj, M.; Bentz, K.C.; Ayala, S., Jr.; Palomba, J.M.; Barcus, K.S.; Katayama, Y.; Cohen, S.M. MOF-polymer hybrid materials: From simple composites to tailored architectures. Chem. Rev. 2020, 120, 8267–8302. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Wang, L.; Peng, X.; Fu, H.; Huang, C.; Li, Y.; Liu, Z. Recent advances in the development of electrochemical aptasensors for detection of heavy metals in food. Biosens. Bioelectron. 2020, 147, 111777. [Google Scholar] [CrossRef]
- Lu, M.; Deng, Y.; Luo, Y.; Lv, J.; Li, T.; Xu, J.; Chen, S.-W.; Wang, J. Graphene aerogel–metal–organic framework-based electrochemical method for simultaneous detection of multiple heavy-metal ions. Anal. Chem. 2018, 91, 888–895. [Google Scholar] [CrossRef]
- Singh, S.; Numan, A.; Zhan, Y.; Singh, V.; Van Hung, T.; Nam, N.D. A novel highly efficient and ultrasensitive electrochemical detection of toxic mercury (II) ions in canned tuna fish and tap water based on a copper metal-organic framework. J. Hazard. Mater. 2020, 399, 123042. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Li, Y.; Li, Y.; Li, Z.; Zhang, W.; Zou, X.; Shi, J.; Huang, X.; Liu, C. Rapid detection of cadmium ions in meat by a multi-walled carbon nanotubes enhanced metal-organic framework modified electrochemical sensor. Food Chem. 2021, 357, 129762. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, W.; Shen, Z.; Sun, S.; Dai, H.; Ma, H.; Lin, M. Sensitive and selective detection of Pb (II) and Cu (II) using a metal-organic framework/polypyrrole nanocomposite functionalized electrode. Sens. Actuators B Chem. 2020, 304, 127286. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Zhu, S.; Yan, M.; Ge, S.; Yu, J. 3D origami electrochemical device for sensitive Pb2+ testing based on DNA functionalized iron-porphyrinic metal-organic framework. Biosens. Bioelectron. 2017, 87, 108–115. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Pournara, A.; Manos, M.; Spanopoulos, I.; Kanatzidis, M.; Tziotzi, T.; Petkov, V.; Margariti, A.; Oikonomopoulos, P. 3D-printed lab-in-a-syringe voltammetric cell based on a working electrode modified with a highly efficient Ca-MOF sorbent for the determination of Hg (II). Sens. Actuators B Chem. 2020, 321, 128508. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Y.; Zhu, M.; Xu, Y.; Guo, Z.; Shi, J.; Han, E.; Zou, X.; Wang, D. Electrochemical DNA sensor for inorganic mercury (II) ion at attomolar level in dairy product using Cu (II)-anchored metal-organic framework as mimetic catalyst. Chem. Eng. J. 2020, 383, 123182. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, M.; Jiang, Y.; Wang, X.; Guo, Z.; Shi, J.; Zou, X.; Han, E. Simple electrochemical sensing for mercury ions in dairy product using optimal Cu2+-based metal-organic frameworks as signal reporting. J. Hazard. Mater. 2020, 400, 123222. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Xu, Y.; Wang, X.; Guo, Z.; Huang, X.; Li, Z.; Shi, J.; Zou, X. Single-step electrochemical sensing of ppt-level lead in leaf vegetables based on peroxidase-mimicking metal-organic framework. Biosens. Bioelectron. 2020, 168, 112544. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, E.A.; Cagliani, L.R.; Tarantilis, P.A.; Polissiou, M.G.; Consonni, R. Sudan dyes in adulterated saffron (Crocus sativus L.): Identification and quantification by 1H NMR. Food Chem. 2017, 217, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Shaffer, R.M.; Sathyanarayana, S.; Lowry, J.A.; Ahdoot, S.; Baum, C.R.; Bernstein, A.S.; Bole, A.; Campbell, C.C.; Landrigan, P.J. Food additives and child health. Pediatrics 2018, 142, e20181410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovina, K.; Siddiquee, S. Analytical and advanced methods-based determination of melamine in food products. In Nanobiosensors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 339–390. [Google Scholar]
- Zhou, Y.; Li, X.; Pan, Z.; Ye, B.; Xu, M. Determination of malachite green in fish by a modified MOF-based electrochemical sensor. Food Anal. Methods 2019, 12, 1246–1254. [Google Scholar] [CrossRef]
- Darabi, R.; Shabani-Nooshabadi, M.; Karimi-Maleh, H.; Gholami, A. The potential of electrochemistry for one-pot and sensitive analysis of patent blue V, tartrazine, acid violet 7 and ponceau 4R in foodstuffs using IL/Cu-BTC MOF modified sensor. Food Chem. 2022, 368, 130811. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Peng, L.; Chen, T.; Li, X.; Zhu, X.; Hu, P. Facile synthesis of Fe-BTC and electrochemical enhancement effect for sunset yellow determination. Talanta Open 2022, 5, 100084. [Google Scholar] [CrossRef]
- Lv, R.; Sun, R.; Du, T.; Li, Y.; Chen, L.; Zhang, Y.; Qi, Y. Cu2+ modified Zr-based metal organic framework-CTAB-graphene for sensitive electrochemical detection of sunset yellow. Food Chem. Toxicol. 2022, 166, 113250. [Google Scholar] [CrossRef]
- Salagare, S.; Shivappa Adarakatti, P.; Venkataramanappa, Y. Designing and construction of carboxyl functionalised MWCNTs/Co-MOFs-based electrochemical sensor for the sensitive detection of nitrite. Int. J. Environ. Anal. Chem. 2022, 102, 5301–5320. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Wen, J.-J.; Xu, H.-X.; Qiu, Y.-B.; Yin, Z.-Z.; Li, L.-H.; Gu, C.-C. Development of a copper-based metal organic electrode for nitrite sensing. J. AOAC Int. 2021, 104, 157–164. [Google Scholar] [CrossRef]
- He, B.; Yan, D. Au/ERGO nanoparticles supported on Cu-based metal-organic framework as a novel sensor for sensitive determination of nitrite. Food Control 2019, 103, 70–77. [Google Scholar] [CrossRef]
- Saeb, E.; Asadpour-Zeynali, K. A novel ZIF-8@ ZIF-67/Au core–shell metal organic framework nanocomposite as a highly sensitive electrochemical sensor for nitrite determination. Electrochim. Acta 2022, 417, 140278. [Google Scholar] [CrossRef]
- Velusamy, V.; Arshak, K.; Korostynska, O.; Oliwa, K.; Adley, C. An overview of foodborne pathogen detection: In the perspective of biosensors. Biotechnol. Adv. 2010, 28, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Tauxe, R.V. Emerging foodborne pathogens. Int. J. Food Microbiol. 2002, 78, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lin, C.-W.; Wang, J.; Oh, D.H. Advances in rapid detection methods for foodborne pathogens. J. Microbiol. Biotechnol. 2014, 24, 297–312. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Yan, X. Ultrasensitive electrochemical aptasensor based on CoSe2/AuNRs and 3D structured DNA-PtNi@ Co-MOF networks for the detection of zearalenone. Sens. Actuators B Chem. 2020, 306, 127558. [Google Scholar] [CrossRef]
- Zhu, X.; Li, B.; Yang, J.; Li, Y.; Zhao, W.; Shi, J.; Gu, J. Effective adsorption and enhanced removal of organophosphorus pesticides from aqueous solution by Zr-based MOFs of UiO-67. ACS Appl. Mater. Interfaces 2015, 7, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, W.; Xia, L.; Cui, C.; Wan, S.; Jiang, Y.; Yang, Y.; Wu, Q.; Qiu, L.; Tan, W. ZrMOF nanoparticles as quenchers to conjugate DNA aptamers for target-induced bioimaging and photodynamic therapy. Chem. Sci. 2018, 9, 7505–7509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, G.; Li, Z.; Luo, F.; Ai, S.; Chen, B.; Wang, Q. Electrochemical determination of Salmonella typhimurium by using aptamer-loaded gold nanoparticles and a composite prepared from a metal-organic framework (type UiO-67) and graphene. Microchim. Acta 2019, 186, 620. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, X.; Meng, Q.; Zheng, P.; Zhang, J.; He, Z.; Jiang, H. Gold interdigitated micro-immunosensor based on Mn-MOF-74 for the detection of Listeria monocytogens. Biosens. Bioelectron. 2021, 183, 113186. [Google Scholar] [CrossRef]
- Lu, X.; He, B.; Liang, Y.; Wang, J.; Jiao, Q.; Liu, Y.; Guo, R.; Wei, M.; Jin, H.; Ren, W. An electrochemical aptasensor based on dual-enzymes-driven target recycling strategy for patulin detection in apple juice. Food Control 2022, 137, 108907. [Google Scholar] [CrossRef]
- Kaur, G.; Sharma, S.; Singh, S.; Bhardwaj, N.; Deep, A. Selective and Sensitive Electrochemical Sensor for Aflatoxin M1 with a Molybdenum Disulfide Quantum Dot/Metal–Organic Framework Nanocomposite. ACS Omega 2022, 7, 17600–17608. [Google Scholar] [CrossRef]
- Jahangiri–Dehaghani, F.; Zare, H.R.; Shekari, Z. Measurement of aflatoxin M1 in powder and pasteurized milk samples by using a label–free electrochemical aptasensor based on platinum nanoparticles loaded on Fe–based metal–organic frameworks. Food Chem. 2020, 310, 125820. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri–Dehaghani, F.; Zare, H.R.; Shekari, Z. A non-label electrochemical aptasensor based on Cu metal–organic framework to measure aflatoxin B1 in wheat flour. Food Anal. Methods 2022, 15, 192–202. [Google Scholar] [CrossRef]
- Feng, K.; Li, T.; Ye, C.; Gao, X.; Yang, T.; Liang, X.; Yue, X.; Ding, S.; Dong, Q.; Yang, M. A label-free electrochemical immunosensor for rapid detection of salmonella in milk by using CoFe-MOFs-graphene modified electrode. Food Control 2021, 130, 108357. [Google Scholar] [CrossRef]
- Duan, F.; Rong, F.; Guo, C.; Chen, K.; Wang, M.; Zhang, Z.; Pettinari, R.; Zhou, L.; Du, M. Electrochemical aptasensing strategy based on a multivariate polymertitanium-metal-organic framework for zearalenone analysis. Food Chem. 2022, 385, 132654. [Google Scholar] [CrossRef]
- Song, Y.; He, L.; Zhang, S.; Liu, X.; Chen, K.; Jia, Q.; Zhang, Z.; Du, M. Novel impedimetric sensing strategy for detecting ochratoxin A based on NH2-MIL-101 (Fe) metal-organic framework doped with cobalt phthalocyanine nanoparticles. Food Chem. 2021, 351, 129248. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, B.; Costa-García, A.; Muñiz, A.d.l.E. Electrochemical (bio) sensors for pesticides detection using screen-printed electrodes. Biosensors 2020, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Authority, E.F.S.; Bellisai, G.; Bernasconi, G.; Brancato, A.; Cabrera, L.C.; Castellan, I.; Ferreira, L.; Giner, G.; Greco, L.; Jarrah, S. Targeted review of maximum residues levels (MRLs) for indoxacarb. EFSA J. 2022, 20, e07527. [Google Scholar]
- Li, X.; Gao, X.; Gai, P.; Liu, X.; Li, F. Degradable metal-organic framework/methylene blue composites-based homogeneous electrochemical strategy for pesticide assay. Sens. Actuators B Chem. 2020, 323, 128701. [Google Scholar] [CrossRef]
- Janjani, P.; Bhardwaj, U.; Gupta, R.; Kushwaha, H.S. Bimetallic Mn/Fe MOF modified screen-printed electrodes for non-enzymatic electrochemical sensing of organophosphate. Anal. Chim. Acta 2022, 1202, 339676. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Fakhri, H.; Hajian, A.; Afkhami, A.; Bagheri, H. High-performance electrochemical enzyme sensor for organophosphate pesticide detection using modified metal-organic framework sensing platforms. Bioelectrochemistry 2019, 130, 107348. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, J.; Wang, B.; Guo, Y.; Dong, X.; Zhao, J. An amino-modified metal-organic framework (type UiO-66-NH2) loaded with cadmium (II) and lead (II) ions for simultaneous electrochemical immunosensing of triazophos and thiacloprid. Microchim. Acta 2019, 186, 101. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Jia, X.; Zhong, L.; Jiao, Y.; Zhang, Z.; Wang, Z.; Feng, Y.; Bilal, M.; Cui, J.; Jia, S. Metal-organic frameworks with different dimensionalities: An ideal host platform for enzyme@ MOF composites. Coord. Chem. Rev. 2022, 454, 214327. [Google Scholar] [CrossRef]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.; Tang, T.-H. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef]
- Ma, L.; He, Y.; Wang, Y.; Wang, Y.; Li, R.; Huang, Z.; Jiang, Y.; Gao, J. Nanocomposites of Pt nanoparticles anchored on UiO66-NH2 as carriers to construct acetylcholinesterase biosensors for organophosphorus pesticide detection. Electrochim. Acta 2019, 318, 525–533. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Rao, R.P.; Mukherjee, I.; Agrawal, P.K.; Basu, T.; Bharadwaj, L.M. Layered construction of nano immuno-hybrid embedded MOF as an electrochemical sensor for rapid quantification of total pesticides load in vegetable extract. J. Electroanal. Chem. 2020, 873, 114386. [Google Scholar]
- Chen, X.; Zhang, Q. Recent advances in mesoporous metal-organic frameworks. Particuology 2019, 45, 20–34. [Google Scholar] [CrossRef]
- Liu, D.; Zou, D.; Zhu, H.; Zhang, J. Mesoporous metal–organic frameworks: Synthetic strategies and emerging applications. Small 2018, 14, 1801454. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, B.; Tan, C.-P.; Lai, O.-M.; Panpipat, W.; Cheong, L.-Z.; Shen, C. Hierarchical macro-microporous ZIF-8 nanostructures as efficient nano-lipase carriers for rapid and direct electrochemical detection of nitrogenous diphenyl ether pesticides. Sens. Actuators B Chem. 2020, 321, 128477. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Shen, C.; Wang, C.; Hu, X.; Wang, G. An electrochemical sensor on the hierarchically porous Cu-BTC MOF platform for glyphosate determination. Sens. Actuators B Chem. 2019, 283, 487–494. [Google Scholar] [CrossRef]
- Hira, S.A.; Annas, D.; Nagappan, S.; Kumar, Y.A.; Song, S.; Kim, H.-J.; Park, S.; Park, K.H. Electrochemical sensor based on nitrogen-enriched metal–organic framework for selective and sensitive detection of hydrazine and hydrogen peroxide. J. Environ. Chem. Eng. 2021, 9, 105182. [Google Scholar] [CrossRef]
- Cheng, D.; Li, P.; Zhu, X.; Liu, M.; Zhang, Y.; Liu, Y. Enzyme-free Electrochemical Detection of Hydrogen Peroxide Based on the Three-Dimensional Flower-like Cu-based Metal Organic Frameworks and MXene Nanosheets. Chin. J. Chem. 2021, 39, 2181–2187. [Google Scholar] [CrossRef]
- Golsheikh, A.M.; Yeap, G.-Y.; Yam, F.K.; San Lim, H. Facile fabrication and enhanced properties of copper-based metal organic framework incorporated with graphene for non-enzymatic detection of hydrogen peroxide. Synth. Met. 2020, 260, 116272. [Google Scholar] [CrossRef]
- Guo, X.; Lin, C.; Zhang, M.; Duan, X.; Dong, X.; Sun, D.; Pan, J.; You, T. 2D/3D Copper-Based Metal-Organic Frameworks for Electrochemical Detection of Hydrogen Peroxide. Front. Chem. 2021, 9, 743637. [Google Scholar] [CrossRef]
- Wang, C.; Huang, S.; Luo, L.; Zhou, Y.; Lu, X.; Zhang, G.; Ye, H.; Gu, J.; Cao, F. Ultrathin two-dimension metal-organic framework nanosheets/multi-walled carbon nanotube composite films for the electrochemical detection of H2O2. J. Electroanal. Chem. 2019, 835, 178–185. [Google Scholar] [CrossRef]
- Yang, J.; Ye, H.; Zhao, F.; Zeng, B. A novel Cu x O nanoparticles@ ZIF-8 composite derived from core–shell metal–organic frameworks for highly selective electrochemical sensing of hydrogen peroxide. ACS Appl. Mater. Interfaces 2016, 8, 20407–20414. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-S.; Li, J.-H.; Chen, Y.-C.; Ho, W.H.; Song, Y.-D.; Kung, C.-W. Electrodeposition of pore-confined cobalt in metal–organic framework thin films toward electrochemical H2O2 detection. Electrochim. Acta 2020, 347, 136276. [Google Scholar] [CrossRef]
- Arul, P.; John, S.A. Silver nanoparticles built-in zinc metal organic framework modified electrode for the selective non-enzymatic determination of H2O2. Electrochim. Acta 2017, 235, 680–689. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Hou, M.; Li, X.; Wu, X.; Ge, J. Immobilization on metal–organic framework engenders high sensitivity for enzymatic electrochemical detection. ACS Appl. Mater. Interfaces 2017, 9, 13831–13836. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, B.; Li, L.; Song, J.; Song, L.; Zhang, M. Fewer-layer conductive metal-organic Langmuir-Blodgett films as electrocatalysts enable an ultralow detection limit of H2O2. Appl. Surf. Sci. 2021, 539, 148255. [Google Scholar] [CrossRef]
- Joshi, A.; Kim, K.-H. Recent advances in nanomaterial-based electrochemical detection of antibiotics: Challenges and future perspectives. Biosens. Bioelectron. 2020, 153, 112046. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, Z.; Verdian, A.; Housaindokht, M.R.; Izadyar, M.; Rouhbakhsh, Z. Aptasensors as the future of antibiotics test kits-a case study of the aptamer application in the chloramphenicol detection. Biosens. Bioelectron. 2018, 122, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Luong, J.H. Handbook of Immunoassay Technologies: Approaches, Performances, and Applications; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Liu, X.; Hu, M.; Wang, M.; Song, Y.; Zhou, N.; He, L.; Zhang, Z. Novel nanoarchitecture of Co-MOF-on-TPN-COF hybrid: Ultralowly sensitive bioplatform of electrochemical aptasensor toward ampicillin. Biosens. Bioelectron. 2019, 123, 59–68. [Google Scholar] [CrossRef]
- Liu, C.-S.; Sun, C.-X.; Tian, J.-Y.; Wang, Z.-W.; Ji, H.-F.; Song, Y.-P.; Zhang, S.; Zhang, Z.-H.; He, L.-H.; Du, M. Highly stable aluminum-based metal-organic frameworks as biosensing platforms for assessment of food safety. Biosens. Bioelectron. 2017, 91, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, M.; Li, Z.; He, L.; Hu, M.; He, L.; Zhang, Z.; Du, M. A bimetallic CoNi-based metal− organic framework as efficient platform for label-free impedimetric sensing toward hazardous substances. Sens. Actuators B Chem. 2020, 311, 127927. [Google Scholar] [CrossRef]
- Zhou, N.; Ma, Y.; Hu, B.; He, L.; Wang, S.; Zhang, Z.; Lu, S. Construction of Ce-MOF@ COF hybrid nanostructure: Label-free aptasensor for the ultrasensitive detection of oxytetracycline residues in aqueous solution environments. Biosens. Bioelectron. 2019, 127, 92–100. [Google Scholar] [CrossRef]
- He, H.; Wang, S.-Q.; Han, Z.-Y.; Tian, X.-H.; Zhang, W.-W.; Li, C.-P.; Du, M. Construction of electrochemical aptasensors with Ag (I) metal− organic frameworks toward high-efficient detection of ultra-trace penicillin. Appl. Surf. Sci. 2020, 531, 147342. [Google Scholar] [CrossRef]
- Song, Y.; Xu, M.; Liu, X.; Li, Z.; Wang, C.; Jia, Q.; Zhang, Z.; Du, M. A label-free enrofloxacin electrochemical aptasensor constructed by a semiconducting CoNi-based metal–organic framework (MOF). Electrochim. Acta 2021, 368, 137609. [Google Scholar] [CrossRef]
- Meng, X.; Gu, H.; Yi, H.; He, Y.; Chen, Y.; Sun, W. Sensitive detection of streptomycin in milk using a hybrid signal enhancement strategy of MOF-based bio-bar code and target recycling. Anal. Chim. Acta 2020, 1125, 1–7. [Google Scholar] [CrossRef]
- Yin, C.; Zhuang, Q.; Xiao, Q.; Wang, Y.; Xie, J. Electropolymerization of poly (methylene blue) on flower-like nickel-based MOFs used for ratiometric electrochemical sensing of total polyphenolic content in chrysanthemum tea. Anal. Methods 2021, 13, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hassan, M.; Bo, X.; Guo, L. Fumarate-based metal-organic framework/mesoporous carbon as a novel electrochemical sensor for the detection of gallic acid and luteolin. J. Electroanal. Chem. 2019, 849, 113378. [Google Scholar] [CrossRef]
- Yan, Y.; Bo, X.; Guo, L. MOF-818 metal-organic framework-reduced graphene oxide/multiwalled carbon nanotubes composite for electrochemical sensitive detection of phenolic acids. Talanta 2020, 218, 121123. [Google Scholar] [CrossRef]
- Ling, W.; Liew, G.; Li, Y.; Hao, Y.; Pan, H.; Wang, H.; Ning, B.; Xu, H.; Huang, X. Materials and techniques for implantable nutrient sensing using flexible sensors integrated with metal–organic frameworks. Adv. Mater. 2018, 30, 1800917. [Google Scholar] [CrossRef]
- Venkadesh, A.; Mathiyarasu, J.; Radhakrishnan, S. Voltammetric Sensing of Caffeine in Food Sample Using Cu-MOF and Graphene. Electroanalysis 2021, 33, 1007–1013. [Google Scholar] [CrossRef]
- Raj, C.R.; Tokuda, K.; Ohsaka, T. Electroanalytical applications of cationic self-assembled monolayers: Square-wave voltammetric determination of dopamine and ascorbate. Bioelectrochemistry 2001, 53, 183–191. [Google Scholar] [CrossRef]
- Gayathri, P.; Ramanujam, K. Redox active cobalt-bipyridine metal organic framework-nafion coated carbon nanotubes for sensing ascorbic acid. J. Electrochem. Soc. 2018, 165, B603. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, S.; Hu, X.; Shao, Z.; Zheng, M.; Pang, H. Ultrathin Nanosheet Ni-Metal Organic Framework Assemblies for High-Efficiency Ascorbic Acid Electrocatalysis. ChemElectroChem 2018, 5, 3859–3865. [Google Scholar] [CrossRef]
- Valizadeh, H.; Tashkhourian, J.; Abbaspour, A. A carbon paste electrode modified with a metal-organic framework of type MIL-101 (Fe) for voltammetric determination of citric acid. Microchim. Acta 2019, 186, 455. [Google Scholar] [CrossRef]
- Freire, C.; Fernandes, D.M.; Nunes, M.; Abdelkader, V.K. POM & MOF-based Electrocatalysts for Energy-related Reactions. ChemCatChem 2018, 10, 1703–1730. [Google Scholar]
- Wang, X.-L.; Song, G.; Lin, H.-Y.; Wang, X.; Liu, G.-C.; Rong, X. Polyoxometalate-induced different metal–organic frameworks based on isonicotinic acid and AgI ion: Syntheses, structures and properties. Inorg. Chem. Commun. 2017, 84, 168–173. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Barbosa, A.D.; Pires, J.; Balula, S.S.; Cunha-Silva, L.; Freire, C. Novel composite material polyoxovanadate@ MIL-101 (Cr): A highly efficient electrocatalyst for ascorbic acid oxidation. ACS Appl. Mater. Interfaces 2013, 5, 13382–13390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gu, C.; Fu, Y.; Liu, L.; Xie, Y. Ultrasensitive electrochemical sensor for luteolin based on zirconium metal-organic framework UiO-66/reduced graphene oxide composite modified glass carbon electrode. Molecules 2020, 25, 4557. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Tu, X.; Ma, X.; Xie, Y.; Zou, J.; Huang, X.; Qu, F.; Yu, Y.; Lu, L. NiO@ Ni-MOF nanoarrays modified Ti mesh as ultrasensitive electrochemical sensing platform for luteolin detection. Talanta 2020, 215, 120891. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Cheng, D.; Li, P.; Xu, Z.; Zhu, X.; Zhang, Y.; Li, H.; Liu, X.; Liu, M.; Yao, S. Au/metal–organic framework nanocapsules for electrochemical determination of glutathione. ACS Appl. Nano Mater. 2021, 4, 4853–4862. [Google Scholar] [CrossRef]



| Number | Electrode Materials | Analyte | Electrochemical Method | Sensitivity | DL | Ref |
|---|---|---|---|---|---|---|
| 1 | GA/UiO-66-NH2 | Heavy Metals | Differential pulse stripping voltammetry (DPSV) | 0.2716, 0.5242, 0.3604, 0.3242 (μA/μM) for (Cd2+, Pb2+, Cu2+, Hg2+) respectively | 0.02 μM for Cd2+, 1.5 nM for Pb2+, 7 nM for Cu2+, and 2 nM for Hg2+ | [35] |
| 2 | Cu-MOF | Hg2+ | Differential pulse voltammetry (DPV) | 0.062 (μA/nM) | 0.0633 nM | [36] |
| 3 | MWCNT/UiO-66-NH2 | Cd2+ | electrochemical impedance spectroscopy (EIS) and CV | 0.11 (μA/μg L−1) | 0.2 μg/L | [37] |
| 4 | NH2-MIL-53(Al)/PPy | Heavy Metals | DPV | 0.21 μA/μg L−1) for Pb2+ 0.1 (μA/μg L−1 for Cu2+ | 0.315 μg L−1 and 0.244 μg L−1 for Pb2+ and Cu2+ respectively | [38] |
| 5 | DNA/(Fe-)n-MOF | Heavy Metals | DPV | 1.16 (μA/nM) | 0.02 nmol L1 | [39] |
| 6 | Ca-MOF | Heavy Metals | anodic stripping voltammetry (ASV | 0.3 μA/μg L | 0.6 µg L−1 | [40] |
| 7 | DNA/Cu-MOF | Heavy Metals | DPV | 0.99 (μA/M) for Hg2+ | 4.8 fM | [42] |
| 8 | DNAzyme/porph@MOF | Heavy Metals | Square wave voltammetric (SWV) | 2.67 (μA/M) for Pb2+ | 5 pM | [43] |
| 9 | Ag/Cu-MOFs | Food additives | DPV | 0.03 (μA/nM) for | 2.2 nM | [47] |
| 11 | Fe-BTC | sunset yellow | DPV | 0.02 (μA/nM) for. | 0.015 nM | [49] |
| 13 | MWCNTs/Co-MOFs | nitrite | DPV | 6.7 (μA/μM) for | 18.8 µM | [51] |
| 14 | Cu-MOF | Nitrite | Amperometry | 7.48 (μA/mM) | 72 nM | [52] |
| 15 | rGO/Cu-TDPAT | nitrite | Amperometry and DPV | 0.0096 and 0.0095 (μA/μM) by amperometry and DPV respectively | 0.006 μmol·L−1 | [53] |
| 16 | 3D structured DNA-PtNi@Co-MOF | (ZEN) | DPV | 3 (μA/g mL−1) for | 1.37 fg/mL | [58] |
| 17 | cDNA/UiO-67-GR | S. typhimurium | DPV | 3.12 (μA/CFU·mL−1) | 5 CFU·mL−1 | [61] |
| 18 | Mn-MOF-74 | Listeria monocytogens | EIS | 1.378(Ω/CFU·mL−1) | 7.1 CFU/mL | [62] |
| 19 | MoS2/UiO-66 | aflatoxin M1 | EIS | 1.27 (kΩ/ng mL−1) for | 0.06 ng mL−1 | [38] |
| 20 | ZIF-8/MB | Pesticide residue | DPV | 0.2 (nA/ng mL−1) ZIF-8/MB | 1.7 ng/mL | [72] |
| 21 | Ce/UiO-66@MWCNTs | acetylthiocholine chloride | Amperometry | 0.015 (μA/μM) | 0.004 nM | [74] |
| 22 | Pt@UiO66-NH2 | malathion | SWV | 270 (μA/μM) for | 4.9 × 10−15 M. | [79] |
| 23 | Chi-AuNP-rIgG-BSA/MOF-5 | chlorpyrifos | CV | 25.4 μA/ng Lcm−2 | 4 (ngL−1) | [80] |
| 24 | MAC-ZIF-8 | nitrofen | CV | 0.03 (μA/μM) | 0.46 µM | [83] |
| 25 | Cu-BTC | glyphosate | DPV | 0.78 (μA/μM) | 1.4 × 10−13 M | [84] |
| 26 | N-Co-MOF | hydrogen peroxide | Amperometry | 0.03 (μA/μM) | 0.072 µM | [85] |
| 27 | MXene/Cu-MOF | hydrogen peroxide | Amperometry | 0.03 (μA/μM) | 0.35 μmol/L | [86] |
| 28 | Cu-BTC-MOF/GO | hydrogen peroxide | Amperometry | 0.02 (mA/mM) | 0.44 μM | [87] |
| 29 | HKUST-1 | hydrogen peroxide | CV | 46.38 (μA/mM) | 0.68 μM | [88] |
| 30 | 2D Cu-TCPP/MWCNT | hydrogen peroxide | Amperometry | 157 μA/cm−2mM−1 | 0.70 μM | [89] |
| 31 | CuxO/Cu3(BTC)2 | hydrogen peroxide | Amperometry | 178 (μA mM−1 cm−2) | 0.15 μM | [90] |
| 32 | Co@MOF-808 | hydrogen peroxide | Amperometry | 382.27 (μA/mM-cm2) | 1.3 μM | [91] |
| 33 | AgNPs-Zn-MOF | hydrogen peroxide | DPV | 0.03 (μA/μM) | 67 nM | [92] |
| 34 | Cytochrome c/ZIF-8 | hydrogen peroxide | Amperometry | 3.84 (mA·M−1·cm −2) | - | [93] |
| 35 | CoNi-MOF | SAL | EIS | 01 kΩ/ng·mL−1 | 0.30 pg·mL−1 | [100] |
| 36 | Ce-MOF@MCA | oxytetracycline | EIS | 051 kΩ/ng·mL−1 | 35.0 fM | [101] |
| 37 | Ag(I)-MOF | penicillin | EIS | 3.69 kΩ/ng·mL−1 | 0.849 pg mL−1 | [102] |
| 38 | CoxNi3-x(HITP)2 | enrofloxacin | EIS | 4.46 kΩ/pg·mL−1 | 0.2 fg·mL−1 | [103] |
| 39 | UiO-66-NH2 | streptomycin | DPV | 1.5 μA/ng mL−1 | 2.6 pg mL−1 | [104] |
| 40 | MOF-801 | luteolin | DPV | 44.363 μA μM−1 | 2.90 nM | [105] |
| 41 | MOF-818@RGO/MWCNTs | chlorogenic acid (CGA), | DPV | 12.50 μA/μM | 5.7 nM | [106] |
| 42 | Cu-MOF/Graphene | Caffeine | LSV | 0.710 μA μM−1 cm−2 | 1.38 μM | [109] |
| 43 | MIL-101(Fe) | CA | DPV | 0.67 μA·μM−1·cm−2 | 4.0 μM | [113] |
| 44 | UiO-66/ErGO | Luteolin | DPV | 0.00075 μM | [117] | |
| 45 | NiO@Ni-MOF | Luteolin | DPV | 25.4 μA/μM | 3 pM | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseinzadeh, B.; Rodriguez-Mendez, M.L. Electrochemical Sensor for Food Monitoring Using Metal-Organic Framework Materials. Chemosensors 2023, 11, 357. https://doi.org/10.3390/chemosensors11070357
Hosseinzadeh B, Rodriguez-Mendez ML. Electrochemical Sensor for Food Monitoring Using Metal-Organic Framework Materials. Chemosensors. 2023; 11(7):357. https://doi.org/10.3390/chemosensors11070357
Chicago/Turabian StyleHosseinzadeh, Batoul, and Maria Luz Rodriguez-Mendez. 2023. "Electrochemical Sensor for Food Monitoring Using Metal-Organic Framework Materials" Chemosensors 11, no. 7: 357. https://doi.org/10.3390/chemosensors11070357