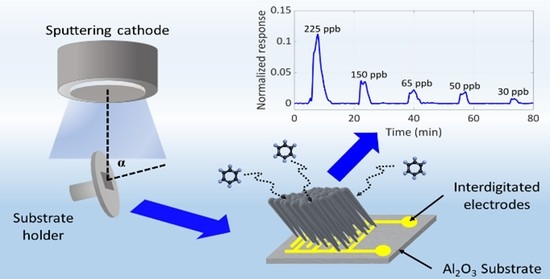
Nanostructuring of SnO2 Thin Films by Associating Glancing Angle Deposition and Sputtering Pressure for Gas Sensing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Growth
2.2. Characterization
2.3. Sensing Tests
3. Results and Discussion
3.1. Morphology, Structure, and Composition of the Films
3.2. Film Growth
3.2.1. Through the Films
3.2.2. Surface Porosity
3.3. Sensing Performances
3.3.1. Benzene Detection
3.3.2. BTEX Discrimination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of Portable and Low-Cost Sensors for the Ambient Air Monitoring of Benzene and Other Volatile Organic Compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhall, S.; Mehta, B.R.; Tyagi, A.K.; Sood, K. A Review on Environmental Gas Sensors: Materials and Technologies. Sens. Int. 2021, 2, 100116. [Google Scholar] [CrossRef]
- Shimizu, Y.; Jono, A.; Hyodo, T.; Egashira, M. Preparation of Large Mesoporous SnO2 Powder for Gas Sensor Application. Sens. Actuators B Chem. 2005, 108, 56–61. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.-D.; Cho, B.K.; Brinzari, V. Grain Size Effects in Sensor Response of Nanostructured SnO2- and In2O3-Based Conductometric Thin Film Gas Sensor. Crit. Rev. Solid State Mater. Sci. 2009, 34, 1–17. [Google Scholar] [CrossRef]
- Kong, Y.; Li, Y.; Cui, X.; Su, L.; Ma, D.; Lai, T.; Yao, L.; Xiao, X.; Wang, Y. SnO2 Nanostructured Materials Used as Gas Sensors for the Detection of Hazardous and Flammable Gases: A Review. Nano Mater. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Sriram, S.R.; Parne, S.; Vaddadi, V.S.C.S.; Edla, D.; Nagaraju, P.; Avala, R.R.; Yelsani, V.; Sontu, U.B. Nanostructured WO3 Based Gas Sensors: A Short Review. Sens. Rev. 2021, 41, 406–424. [Google Scholar] [CrossRef]
- Pant, B.R.; Jayatissa, A.H. Gas Sensor Application of Zinc Oxide. In Chemical Methods for Processing Nanomaterials; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-0-429-02318-7. [Google Scholar]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas Sensors Based on TiO2 Nanostructured Materials for the Detection of Hazardous Gases: A Review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Seekaew, Y.; Wisitsoraat, A.; Phokharatkul, D.; Wongchoosuk, C. Room Temperature Toluene Gas Sensor Based on TiO2 Nanoparticles Decorated 3D Graphene-Carbon Nanotube Nanostructures. Sens. Actuators B Chem. 2019, 279, 69–78. [Google Scholar] [CrossRef]
- Subbiah, D.K.; Mani, G.K.; Babu, K.J.; Das, A.; Balaguru Rayappan, J.B. Nanostructured ZnO on Cotton Fabrics—A Novel Flexible Gas Sensor & UV Filter. J. Clean. Prod. 2018, 194, 372–382. [Google Scholar] [CrossRef]
- Nemec, P.; Hotový, I.; Andok, R.; Kostič, I. Increased Sensitivity of a Gas Sensor by Controlled Extension of TiO2 Active Area. AIP Conf. Proc. 2018, 1996, 020032. [Google Scholar] [CrossRef]
- Atanasova, G.; Dikovska, A.O.; Dilova, T.; Georgieva, B.; Avdeev, G.V.; Stefanov, P.; Nedyalkov, N.N. Metal-Oxide Nanostructures Produced by PLD in Open Air for Gas Sensor Applications. Appl. Surf. Sci. 2019, 470, 861–869. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Tomar, M.; Gupta, V. Tunable Nanostructured Columnar Growth of SnO2 for Efficient Detection of CO Gas. Nanotechnology 2018, 29, 065502. [Google Scholar] [CrossRef] [PubMed]
- Nemec, P.; Hotovy, I.; Rehacek, V.; Andok, R. TiO2 Sensoric Structures with Controlled Extension of Their Active Area by Electron-Beam Lithography and Reactive Ion Etching Techniques. AIP Conf. Proc. 2021, 2411, 060003. [Google Scholar] [CrossRef]
- Bairagi, S.; Järrendahl, K.; Eriksson, F.; Hultman, L.; Birch, J.; Hsiao, C.-L. Glancing Angle Deposition and Growth Mechanism of Inclined AlN Nanostructures Using Reactive Magnetron Sputtering. Coatings 2020, 10, 768. [Google Scholar] [CrossRef]
- El Beainou, R.; Garcia-Valenzuela, A.; Raschetti, M.; Cote, J.-M.; Alvarez, R.; Palmero, A.; Potin, V.; Martin, N. A 4-View Imaging to Reveal Microstructural Differences in Obliquely Sputter-Deposited Tungsten Films. Mater. Lett. 2020, 264, 127381. [Google Scholar] [CrossRef]
- Shin, S.J.; Bayu Aji, L.B.; Bae, J.H.; Engwall, A.M.; Nielsen, M.H.; Hammons, J.A.; Zuo, X.B.; Lee, B.; Lepro, X.; Mirkarimi, P.B.; et al. Oblique Angle Deposition of Boron Carbide Films by Magnetron Sputtering. J. Appl. Phys. 2021, 130, 125305. [Google Scholar] [CrossRef]
- Liedtke, S.; Grüner, C.; Lotnyk, A.; Rauschenbach, B. Glancing Angle Deposition of Sculptured Thin Metal Films at Room Temperature. Nanotechnology 2017, 28, 385604. [Google Scholar] [CrossRef]
- Pedrosa, P.; Ferreira, A.; Martin, N.; Yazdi, M.A.P.; Billard, A.; Lanceros-Méndez, S.; Vaz, F. Nano-Sculptured Janus-like TiAg Thin Films Obliquely Deposited by GLAD Co-Sputtering for Temperature Sensing. Nanotechnology 2018, 29, 355706. [Google Scholar] [CrossRef]
- Sakkas, C.; Rauch, J.-Y.; Cote, J.-M.; Tissot, V.; Gavoille, J.; Martin, N. Tuning the Optical Properties of WO3 Films Exhibiting a Zigzag Columnar Microstructure. Coatings 2021, 11, 438. [Google Scholar] [CrossRef]
- Rydosz, A.; Dyndał, K.; Andrysiewicz, W.; Grochala, D.; Marszałek, K. GLAD Magnetron Sputtered Ultra-Thin Copper Oxide Films for Gas-Sensing Application. Coatings 2020, 10, 378. [Google Scholar] [CrossRef]
- Luo, P.; Xie, M.; Luo, J.; Kan, H.; Wei, Q. Nitric Oxide Sensors Using Nanospiral ZnO Thin Film Deposited by GLAD for Application to Exhaled Human Breath. RSC Adv. 2020, 10, 14877–14884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.G.; Shim, Y.-S.; Han, S.D.; Lee, H.R.; Ju, B.-K.; Kang, C.Y. Metal Oxide Nanocolumns for Extremely Sensitive Gas Sensors. J. Sens. Sci. Technol. 2016, 25, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Cho, I.; Park, J.; Jeong, J.; Lee, K.; Lee, B.; Henriquez, D.D.O.; Yoon, K.; Park, I. High Accuracy Real-Time Multi-Gas Identification by a Batch-Uniform Gas Sensor Array and Deep Learning Algorithm. ACS Sens. 2022, 7, 430–440. [Google Scholar] [CrossRef]
- Davidson, C.J.; Hannigan, J.H.; Bowen, S.E. Effects of Inhaled Combined Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX): Toward an Environmental Exposure Model. Environ. Toxicol. Pharmacol. 2021, 81, 103518. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Arab Pour Yazdi, M.; Sanchez, J.-B.; Billard, A.; Berger, F.; Martin, N. Exploiting the Dodecane and Ozone Sensing Capabilities of Nanostructured Tungsten Oxide Films. Sens. Actuators B Chem. 2018, 266, 773–783. [Google Scholar] [CrossRef]
- Pedrosa, P.; Ferreira, A.; Cote, J.-M.; Martin, N.; Yazdi, M.A.P.; Billard, A.; Lanceros-Mendez, S.; Vaz, F. Influence of the Sputtering Pressure on the Morphological Features and Electrical Resistivity Anisotropy of Nanostructured Titanium Films. Appl. Surf. Sci. 2017, 420, 681–690. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and Collaborative Approach to Problem Solving Using X-Ray Photoelectron Spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Chen, L.-C.; Chen, C.-C.; Sung, Y.-T.; Hsu, Y.-Y. Oblique-Angle Sputtering Effects on Characteristics of Nanocolumnar Structure Anisotropic Indium Tin Oxide Films. J. Electrochem. Soc. 2009, 156, H471. [Google Scholar] [CrossRef]
- Bolzan, A.A.; Fong, C.; Kennedy, B.J.; Howard, C.J. Structural Studies of Rutile-Type Metal Dioxides. Acta Crystallogr. B 1997, 53, 373–380. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Mohammed, A.K.; Sumaila, A. Comparative Study of Crystallite Size Using Williamson-Hall and Debye-Scherrer Plots for ZnO Nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 045013. [Google Scholar] [CrossRef]
- Güzelçimen, F.; Tanören, B.; Çetinkaya, Ç.; Kaya, M.D.; Efkere, H.İ.; Özen, Y.; Bingöl, D.; Sirkeci, M.; Kınacı, B.; Ünlü, M.B.; et al. The Effect of Thickness on Surface Structure of Rf Sputtered TiO2 Thin Films by XPS, SEM/EDS, AFM and SAM. Vacuum 2020, 182, 109766. [Google Scholar] [CrossRef]
- Kövér, L.; Kovács, Z.; Sanjinés, R.; Moretti, G.; Cserny, I.; Margaritondo, G.; Pálinkás, J.; Adachi, H. Electronic Structure of Tin Oxides: High-Resolution Study of XPS and Auger Spectra. Surf. Interface Anal. 1995, 23, 461–466. [Google Scholar] [CrossRef]
- Ohlídal, I.; Vohánka, J.; Čermák, M. Optics of Inhomogeneous Thin Films with Defects: Application to Optical Characterization. Coatings 2020, 11, 22. [Google Scholar] [CrossRef]
- Sánchez-González, J.; Díaz-Parralejo, A.; Ortiz, A.L.; Guiberteau, F. Determination of Optical Properties in Nanostructured Thin Films Using the Swanepoel Method. Appl. Surf. Sci. 2006, 252, 6013–6017. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Zhang, R.-J.; Lu, H.-L.; Chen, X.; Sun, Y.; Zhang, Y.; Wei, Y.-F.; Xu, J.-P.; Wang, S.-Y.; Zheng, Y.-X.; et al. The Impact of Thickness and Thermal Annealing on Refractive Index for Aluminum Oxide Thin Films Deposited by Atomic Layer Deposition. Nanoscale Res. Lett. 2015, 10, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raoult, E.; Bodeux, R.; Jutteau, S.; Rives, S.; Yaiche, A.; Coutancier, D.; Rousset, J.; Collin, S. Optical characterizations and modelling of semitransparent perovskite solar cells for tandem applications. In Proceedings of the 36th European Photovoltaic Solar Energy Conference and Exhibition, Marseille, France, 9–13 September 2019; pp. 757–763. [Google Scholar] [CrossRef]
- Robbie, K.; Brett, M.J. Sculptured Thin Films and Glancing Angle Deposition: Growth Mechanics and Applications. J. Vac. Sci. Technol. Vac. Surf. Films 1997, 15, 1460–1465. [Google Scholar] [CrossRef]
- Sharma, A.; Tomar, M.; Gupta, V. SnO2 Thin Film Sensor with Enhanced Response for NO2 Gas at Lower Temperatures. Sens. Actuators B Chem. 2011, 156, 743–752. [Google Scholar] [CrossRef]
- Bagga, S.; Akhtar, J.; Mishra, S. Influence of Porosity on the Properties of Nanostructured Tin Oxide Thin Film. Mater. Res. Express 2018, 5, 116406. [Google Scholar] [CrossRef]
- Tang, Z.; Chan, P.C.H.; Sharma, R.K.; Yan, G.; Hsing, I.-M.; Sin, J.K.O. Investigation and Control of Microcracks in Tin Oxide Gas Sensing Thin-Films. Sens. Actuators B Chem. 2001, 79, 39–47. [Google Scholar] [CrossRef]
- Sonder, E.; Levinson, L.M.; Katz, W. Role of Short-circuiting Pathways in Reduced ZnO Varistors. J. Appl. Phys. 1985, 58, 4420–4425. [Google Scholar] [CrossRef]
- Filipovic, L.; Selberherr, S. Performance and Stress Analysis of Metal Oxide Films for CMOS-Integrated Gas Sensors. Sensors 2015, 15, 7206–7227. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, A.; Salehi, S. Dynamic Modeling of the Formation Damage and Mud Cake Deposition Using Filtration Theories Coupled with SEM Image Processing. J. Nat. Gas Sci. Eng. 2017, 42, 157–168. [Google Scholar] [CrossRef]
- Kohl, D. Surface Processes in the Detection of Reducing Gases with SnO2-Based Devices. Sens. Actuators 1989, 18, 71–113. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Resistive-Based Gas Sensors for Detection of Benzene, Toluene and Xylene (BTX) Gases: A Review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Xu, C.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain Size Effects on Gas Sensitivity of Porous SnO2-Based Elements. Sens. Actuators B Chem. 1991, 3, 147–155. [Google Scholar] [CrossRef]
- Akbar, M.A.; Ait Si Ali, A.; Amira, A.; Bensaali, F.; Benammar, M.; Hassan, M.; Bermak, A. An Empirical Study for PCA- and LDA-Based Feature Reduction for Gas Identification. IEEE Sens. J. 2016, 16, 5734–5746. [Google Scholar] [CrossRef]
- Liu, H.; Meng, G.; Deng, Z.; Nagashima, K.; Wang, S.; Dai, T.; Li, L.; Yanagida, T.; Fang, X. Discriminating BTX Molecules by the Nonselective Metal Oxide Sensor-Based Smart Sensing System. ACS Sens. 2021, 6, 4167–4175. [Google Scholar] [CrossRef]
- Cho, B.; Lee, K.; Pyo, S.; Kim, J. Fabrication and Characterization of VOC Sensor Array Based on SnO2 and ZnO Nanoparticles Functionalized by Metalloporphyrins. Micro Nano Syst. Lett. 2018, 6, 10. [Google Scholar] [CrossRef]
- Lee, D.-S.; Kim, Y.T.; Huh, J.-S.; Lee, D.-D. Fabrication and Characteristics of SnO2 Gas Sensor Array for Volatile Organic Compounds Recognition. Thin Solid Films 2002, 416, 271–278. [Google Scholar] [CrossRef]







| Lattice Parameters (±0.003 Å) | Average Crystallite Size (±0.1 nm) | Surface Concentration (±0.5 at. %) | Refractive Index at 470 nm (±0.01) | Packing Density (±2%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| a = b | c | Scherrer | Williamson-Hall | O | Sn | ||||
| C3 | 25 °C | 4.783 | 3.217 | 3.0 | 2.8 | - | - | 1.97 | 100 |
| 350 °C | 4.780 | 3.217 | 4.8 | 4.6 | - | - | 1.91 | 97 | |
| 500 °C | 4.761 | 3.186 | 6.9 | 6.7 | 71.1 | 28.9 | 1.81 | 93 | |
| C6 | 25 °C | - | - | - | - | - | - | 1.91 | - |
| 350 °C | - | - | - | - | - | - | 1.93 | - | |
| 500 °C | 4.782 | 3.189 | 1.7 | 1.3 | 67.6 | 32.4 | 1.85 | 92 | |
| I3 | 25 °C | 4.774 | 3.203 | 4.6 | 5.1 | - | - | 1.91 | 97 |
| 350 °C | 4.766 | 3.196 | 5.6 | 5.7 | - | - | 1.89 | 95 | |
| 500 °C | 4.756 | 3.191 | 5.4 | 5.1 | 67.3 | 32.7 | 1.83 | 91 | |
| I6 | 25 °C | - | - | - | - | - | - | 1.80 | - |
| 350 °C | 4.766 | 3.190 | - | - | - | - | 1.79 | 89 | |
| 500 °C | 4.749 | 3.192 | 1.4 | 1.4 | 67.6 | 32.4 | 1.73 | 84 | |
| Materials | Number of Sensors Used | BTEX Concentrations Tested | Ref. |
|---|---|---|---|
| SnO2 | 4 | 50–900 ppb (BTEX) | This study |
| NiO, WO3, SnO2 | 3 | 30–80 ppm (BTX) | [49] |
| SnO2 NPs/cobalt-porphyrin, SnO2 NPs/zinc-porphyrin, SnO2 NPs/nickel-porphyrin and ZnO NPs/cobalt-porphyrin | 4 | 1–9 ppm (BTEX) | [50] |
| SnO2 with several additives, including Pt, Pd, CuO, LaO, ScO, TiO, WO or ZnO | 10 | benzene 50 ppm toluene 500 ppm | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohajir, A.E.; Yazdi, M.A.P.; Krystianiak, A.; Heintz, O.; Martin, N.; Berger, F.; Sanchez, J.-B. Nanostructuring of SnO2 Thin Films by Associating Glancing Angle Deposition and Sputtering Pressure for Gas Sensing Applications. Chemosensors 2022, 10, 426. https://doi.org/10.3390/chemosensors10100426
Mohajir AE, Yazdi MAP, Krystianiak A, Heintz O, Martin N, Berger F, Sanchez J-B. Nanostructuring of SnO2 Thin Films by Associating Glancing Angle Deposition and Sputtering Pressure for Gas Sensing Applications. Chemosensors. 2022; 10(10):426. https://doi.org/10.3390/chemosensors10100426
Chicago/Turabian StyleMohajir, Achraf El, Mohammad Arab Pour Yazdi, Anna Krystianiak, Olivier Heintz, Nicolas Martin, Franck Berger, and Jean-Baptiste Sanchez. 2022. "Nanostructuring of SnO2 Thin Films by Associating Glancing Angle Deposition and Sputtering Pressure for Gas Sensing Applications" Chemosensors 10, no. 10: 426. https://doi.org/10.3390/chemosensors10100426