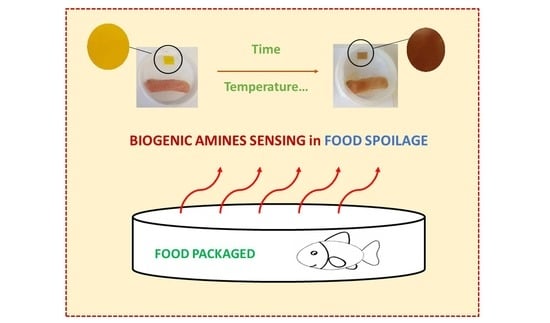
Reversible Colorimetric and Fluorescence Solid Sensors Based on Aryl Hydrazone Derivatives of 1,8-Naphthalimides for Caustic Media and Biogenic Amine Vapors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of the Aryl Hydrazone Derivatives of 1,8-Naphthalimide
2.2.1. Synthesis of N-(2-Hydroxyethyl)-4-(Hydrazine-1-Yl)-1,8-Naphthalimide (Compound 3)
2.2.2. Synthesis of N-(2-Hydroxyethyl)-4-(Benzaldehydehydrazone-1-Yl) -1,8-Naphthalimide (NBH)
2.2.3. Synthesis of N-(2-Hydroxyethyl)-4-(Salicylidene Hydrazone-1-Yl)-1,8-Naphthalimide (NSH)
2.2.4. Synthesis of N-(2-Hydroxyethyl)-4-((Meta-Hydroxy)Benzylidene Hydrazone-1-Yl)-1,8-Naphthalimide N(mHBH)
2.2.5. Synthesis of N-(2-Hydroxyethyl)-4-((Para-Hydroxy)Benzylidene Hydrazone-1-Yl)-1,8-Naphthalimide N(pHBH)
2.3. Membrane Preparation by Photopolymerization (M-Cl) and Functionalization with NBH and NSH
2.4. Characterization and Procedures
3. Results
3.1. Synthesis of Hydrazone Derivatives of Naphthalimides (NBH, NSH, N(Mhbh) and N(pHBH))
3.2. Membrane Preparation, Functionalization with Aryl Hydrazone Derivatives of Naphthalimide Sensors and Characterization
3.3. Effect of pH on the Absorption and Fluorescence Properties of Aryl Hydrazone Derivatives of Naphthalimides and the M-NBH and M-NSH Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, Y.; Zhang, Y.; Xu, H. A Selective “Turn-On” Fluorescent Probe for Recognition of Mercury(II) Ions in Aqueous Solution Based on a Desulfurization Reaction. ChemPlusChem 2013, 78, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Poddar, M.; Sivakumar, G.; Misra, R. Donor–acceptor substituted 1,8-naphthalimides: Design, synthesis, and structure–property relationship. J. Mater. Chem. C 2019, 7, 14798–14815. [Google Scholar] [CrossRef]
- Gudeika, D. A review of investigation on 4-substituted 1,8-naphthalimide derivatives. Synth. Met. 2020, 262, 116328. [Google Scholar] [CrossRef]
- Jain, N.; Kaur, N. A comprehensive compendium of literature of 1,8-Naphthalimide based chemosensors from 2017 to 2021. Coord. Chem. Rev. 2022, 459, 214454. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Tuzi, A.; Piotto, S.; Concilio, S.; Caruso, U. An Amphiphilic Pyridinoyl-hydrazone Probe for Colorimetric and Fluorescence pH Sensing. Molecules 2019, 24, 3833–3855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gemma, S.; Colombo, L.; Forloni, G.; Savini, L.; Fracasso, C.; Caccia, S.; Salmona, M.; Brindisi, M.; Joshi, B.P.; Tripaldi, P.; et al. Pyrroloquinoxaline hydrazones as fluorescent probes for amyloid fibrils. Org. Biomol. Chem. 2011, 9, 5137–5148. [Google Scholar] [CrossRef]
- Shao, B.; Baroncini, M.; Qian, H.; Bussotti, L.; Di Donato, M.; Credi, A.; Aprahamian, I. Solution and Solid-State Emission Toggling of a Photochromic Hydrazone. J. Am. Chem. Soc. 2018, 140, 12323–12327. [Google Scholar] [CrossRef] [Green Version]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydrazone linkages in pH responsive drug delivery systems. Eur. J. Pharm. Sci. 2017, 99, 45–65. [Google Scholar] [CrossRef]
- Pereira, T.M.; Kümmerle, A.E. Computational Biology and Chemistry. Chapter Hydrazone-Based Small-Molecule Chemosensors; IntechOpen: London, UK, 2020. [Google Scholar]
- Zhou, Y.; Piergentili, I.; Hong, J.; Van der Helm, M.P.; Macchione, M.; Li, Y.; Eelkema, R.; Luo, S. Indoline Catalyzed Acylhydrazone/Oxime Condensation under Neutral Aqueous Conditions. Org. Lett. 2020, 22, 6035–6040. [Google Scholar] [CrossRef]
- Shao, B.; Aprahamian, I. pH-Induced Fluorescence and Thermal Relaxation Rate Modulation in a Hydrazone Photoswitch. ChemPhotoChem 2019, 3, 361–364. [Google Scholar] [CrossRef]
- Fan, L.; Qin, J.; Li, T.; Wang, B.; Yangn, Z.A. Chromone Schiff-base as Al(III) selective fluorescent and colorimetric chemosensor. J. Lumin. 2014, 155, 84–88. [Google Scholar] [CrossRef]
- Qin, J.; Yang, Z.; Fan, L.; Cheng, X.; Li, T.; Wang, B. Design and synthesis of a chemosensor for the detection of Al3+ based on ESIPT. Anal. Methods 2014, 6, 7343–7348. [Google Scholar] [CrossRef]
- Kima, S.; Kob, C.; Lima, T.; Yooa, S.; Hamc, H.J.; Kangd, S.-Y.; Kanga, S.; Chob, S.K.; Hana, M. A hydrazone-based turn-on fluorescent probe for peroxynitrite detection and live-cell imaging. Dye. Pigment. 2019, 171, 107762–107768. [Google Scholar] [CrossRef]
- Cheshmedzhieva, D.; Ivanova, P.; Stoyanov, S.; Tasheva, D.; Dimitrova, M.; Ivanovc, I.; Ilieva, S. Experimental and theoretical study on the absorption and fluorescence properties of substituted aryl hydrazones of 1,8-naphthalimide. Phys. Chem. Chem. Phys. 2011, 13, 18530–18538. [Google Scholar] [CrossRef] [PubMed]
- Dilek, O.; Bane, S.L. Synthesis and Spectroscopic Characterization of Fluorescent Boron Dipyrromethene-Derived Hydrazones. J. Fluoresc. 2011, 21, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aysha, T.; Zain, M.; Arief, M.; Youssef, Y. Synthesis and spectral properties of new fluorescent hydrazone disperse dyes and their dyeing application on polyester fabrics. Heliyon 2019, 5, e02358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, Y.; Tong, A.; Jin, P.; Ju, Y. New Fluorescent Rhodamine Hydrazone Chemosensor for Cu(II) with High Selectivity and Sensitivity. Org. Lett. 2006, 8, 2863–2866. [Google Scholar] [CrossRef]
- Pablos, J.L.; Vallejos, S.; Muñoz, A.; Rojo, M.J.; Serna, F.; García, F.C.; García, J.M. Solid Polymer Substrates and Coated Fibers Containing 2,4,6-Trinitrobenzene Motifs as Smart Labels for the Visual Detection of Biogenic Amine Vapors. Chem. Eur. J. 2015, 21, 8733–8736. [Google Scholar] [CrossRef]
- Qi, P.; Wu, X.; Liu, L.; Yu, H.; Song, S. Hydrazone-Containing Triblock Copolymeric Micelles for pH-Controlled Drug Delivery. Front. Pharmacol. 2018, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Zhang, D.; Zhang, Y.; Lu, W.; Zhang, J.; Wang, W.; He, Q.; Théato, P.; Chen, T. Aggregation-Caused Quenching-Type Naphthalimide Fluorophores Grafted and Ionized in a 3D Polymeric Hydrogel Network for Highly Fluorescent and Locally Tunable Emission. ACS Macro Lett. 2019, 8, 937–942. [Google Scholar] [CrossRef]
- Grabchev, I.; Qian, X.; Xiao, Y.; Zhang, R. Novel heterogeneous PET fluorescent sensors selective for transition metal ions or protons: Polymers regularly labelled with naphthalimide. New J. Chem. 2002, 26, 920–925. [Google Scholar] [CrossRef]
- Jiang, J.; Xiao, X.; Zhao, P.; Tian, H. Colorimetric naked-eye recognizable anion sensors synthesized via RAFT polymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1551–1556. [Google Scholar] [CrossRef]
- Fernández-Alonso, S.; Corrales, T.; Pablos, J.L.; Catalina, F. Solid fluorescence sensors obtained by functionalization of photocrosslinked water-swollen acrylic membranes with 4-piperazine naphthalimide derivatives. Polymer 2017, 124, 139–150. [Google Scholar] [CrossRef]
- Pablos, J.L.; Hernández, E.; Catalina, F.; Corrales, T. Solid Fluorescence pH Sensors Based on 1,8-Naphthalimide Copolymers Synthesized by UV Curing. Chemosensors 2022, 10, 73. [Google Scholar] [CrossRef]
- Fernández-Alonso, S.; Corrales, T.; Pablos, J.L.; Catalina, F. A Switchable fluorescence solid sensor for Hg2+ detection in aqueous media based on a photocrosslinked membrane functionalized with (benzimidazolyl)methyl-piperazine derivative of 1,8-naphthalimide. Sens. Actuators B Chem. 2018, 270, 256–2621. [Google Scholar] [CrossRef]
- Fernández-Alonso, S.; Corrales, T.; Pablos, J.L.; Catalina, F. Surface modification of poly(ethylene-butyl acrylate) copolymers by microwave methodology and functionalization with 4-dimethylamino-N-(2-hydroxyethyl)-1,8-naphthalimide for acidity sensing. React. Funct. Polym. 2016, 107, 78–861. [Google Scholar] [CrossRef]
- Allen, N.S.; Corrales, T.; Edge, M.; Catalina, F.; Blanco-Pina, M.; Green, A. Photochemistry and photopolymerization activities of novel phenylthiobenzophenone and diphenylthiophene photoinitiators. Polymer 1998, 39, 903–910. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Shumway, B.R.; Youngbull, C.; Jen, A.K.Y.; Johnson, R.H.; Meldrum, D.R. Dually fluorescent sensing of pH and dissolved oxygen using a membrane made from polymerizable sensing monomers. Sens. Actuators B Chem. 2010, 147, 714–722. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Tang, Y.; Verwilst, P.; Lin, W.; Kim, J.S. A biotin-guided formaldehyde sensor selectively detecting endogenous concentrations in cancerous cells and tissues. Chem. Commun. 2016, 52, 11247–11250. [Google Scholar] [CrossRef]
- Pablos, J.L.; Catalina, F.; Ibeas, S.; Corrales, T. Fluorescent imidazolium-based poly(ionic liquid)s for Fe3+ detection in aqueous medium. J. Photochem. Photobiol. A Chem. 2021, 406, 113015–113024. [Google Scholar] [CrossRef]
- Reijenga, J.; Van Hoof, A.; Van Loon, A.; Teunissen, B. Development of Methods for the Determination of pKa Values. Anal. Chem. Insights 2013, 8, 53–71. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, G.; Drexhage, K. New coumarin dyes with rigidized structure for flash lamp-pumped dye lasers. Opt. Commun. 1975, 13, 222–225. [Google Scholar] [CrossRef]
- Redondo-Foj, B.; Carsí, M.; Ortiz-Serna, P.; Sanchis, M.J.; Vallejos, S.; García, F.; García, J.M. Effect of the Dipole-Dipole interactions in the molecular dynamics of poly(vinylpyrrolidone)-based copolymers. Macromolecules 2014, 47, 5334–5346. [Google Scholar] [CrossRef]
- Niu, C.-G.; Zeng, G.-M.; Chen, L.-X.; Shen, G.-L.; Yu, R.-Q. Proton “off-on” behaviour of methylpiperazinyl derivative of naphthalimide: A pH sensor based on fluorescence enhancement. Analyst 2004, 129, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, S.; Muñoz, A.; Ibeas, S.; Serna, F.; Garcia, F.C.; Garcia, J.M. Solid sensory polymer substrates for the quantification of iron in blood, wine and water by a scalable RGB technique. J. Mater. Chem. A 2013, 1, 15435–15441. [Google Scholar] [CrossRef]










| Parameter | NBH | NSH | N(mHBH) | N(pHBH) | M-NBH | M-NSH |
|---|---|---|---|---|---|---|
| λABS-aci | 326, 465 nm | 330, 460 nm | 330, 465 nm | 330, 475 nm | 460 nm | 460 nm |
| Log εacid(b) | 4.28 (326 nm)/ 4.58 (465 nm) | 4.20 (330 nm)/ 4.52 (460 nm) | 3.95 (330 nm)/ 4.38 (475 nm) | 3.84 (330 nm)/ 4.34 (465 nm) | - | - |
| λIsobestic | 348, 400, 503 nm | 365, 400, 530 nm | 323, 414, 524 nm | 336, 405, 504 nm | 417, 500 nm | 410, 500 nm |
| λABS-base | 367, 565 nm | 386, 568 nm | 384, 566 nm | 377, 575 nm | 583 nm | 576 nm |
| Log εbase(b) | 4.00 (367 nm)/ 4.45 (565 nm) | 4.17 (386 nm)/ 4.5 (568 nm) | 4.14 (384 nm)/ 4.46 (573 nm) | 3.84 (377 nm)/ 4.38 (565 nm) | - | - |
| pKa(c) | 11.7 | 11.5 | 11 | 11.3 | 11.5 | 10.4 |
| λFLU-acid | 562 nm | 560 nm | 548 nm | 561 nm | 525 nm | 533 nm |
| ϕFLU-acid(d) | 0.10 | 0.11 | 0.092 | 0.095 | - | |
| ϕFLU pH=7(d) | 0.090 | 0.098 | 0.092 | 0.095 | - | |
| ϕFLU ethanol(d) | 0.099 | 0.11 | 0.13 | 0.11 | - | |
| ϕFLU hexane(d) | 0.17 | 0.2 | 0.21 | 0.19 | ||
| λFLU-base | 558 nm | 550 nm | 544 nm | 544 nm | 525 nm | 525 nm |
| ϕFLU-base(d) | 0.003 | 0.003 | 0.002 | 0.002 | - | - |
| pKa *(c) | 10.8 | 10.2 | 10.3 | 10.2 | 10.9 | 10 |
| Δν (e) | 3650 | 3560 | 2700 | 3684 | 2700 | 2980 |
| FE(f) | 28.5 | 31.2 | 11.4 | 39 | 8.2 | >40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pablos, J.L.; Fernández-Alonso, S.; Catalina, F.; Corrales, T. Reversible Colorimetric and Fluorescence Solid Sensors Based on Aryl Hydrazone Derivatives of 1,8-Naphthalimides for Caustic Media and Biogenic Amine Vapors. Chemosensors 2022, 10, 417. https://doi.org/10.3390/chemosensors10100417
Pablos JL, Fernández-Alonso S, Catalina F, Corrales T. Reversible Colorimetric and Fluorescence Solid Sensors Based on Aryl Hydrazone Derivatives of 1,8-Naphthalimides for Caustic Media and Biogenic Amine Vapors. Chemosensors. 2022; 10(10):417. https://doi.org/10.3390/chemosensors10100417
Chicago/Turabian StylePablos, Jesús L., Sabela Fernández-Alonso, Fernando Catalina, and Teresa Corrales. 2022. "Reversible Colorimetric and Fluorescence Solid Sensors Based on Aryl Hydrazone Derivatives of 1,8-Naphthalimides for Caustic Media and Biogenic Amine Vapors" Chemosensors 10, no. 10: 417. https://doi.org/10.3390/chemosensors10100417