Cytotoxicity, Phytochemical, Antiparasitic Screening, and Antioxidant Activities of Mucuna pruriens (Fabaceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
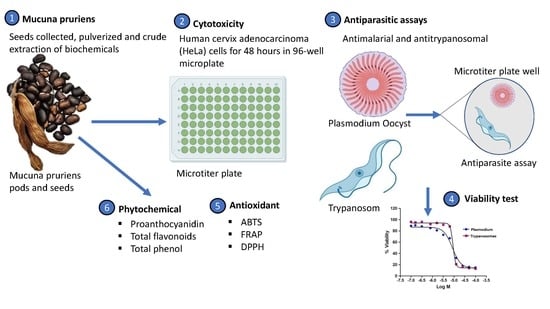
2.2. Crude Extraction
2.3. Cytotoxicity Assay
2.4. Antimalarial Assay
2.5. Trypanocidal Assay
2.6. Phytochemical Screening
2.6.1. Total Phenol
2.6.2. Total Flavonoids
2.6.3. Proanthocyanidin Content (Condensed Tannin)
2.7. Antioxidant Assays
2.7.1. Free Radical Scavenging Activity Using 2,2-Diphenyl-1-picrylhydrazyl (DPPH)
2.7.2. Ferric Reducing Antioxidant Power (FRAP)
2.7.3. Assay with 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS)
2.8. Statistical Analysis
3. Results
3.1. Cytotoxic Effect
3.2. Antimalarial (pLDH) Activity
3.3. Trypanocidal Effect
3.4. Phytochemical Screening
3.5. Percentage DPPH Scavenging Activity
3.6. Ferric Reducing Antioxidant Power
3.7. ABTS Free Radical Scavenging Capacity
4. Discussion
5. Conclusion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| CE | Catechin equivalent |
| FRAP | Ferric reducing antioxidant power |
| GAE | Gallic acid equivalent |
| L-DOPA | L-3,4-Dihydroxyphenylalanine |
| NBT | Nitro blue tetrazolium chloride solution |
| PES | Polyethersulfone solution |
| pLDH | Parasite lactate dehydrogenase |
| QE | Quercetin equivalent |
| RPMI | Roswell Park Memorial Institute |
References
- Lampariello, L.R.; Cortelazzo, A.; Guerranti, R.; Sticozzi, C.; Valacchi, G. The magic velvet bean of Mucuna pruriens. J. Tradit. Complement. Med. 2011, 2, 331–339. [Google Scholar] [CrossRef]
- Tan, N.H.; Fung, S.Y.; Sim, S.M. Extracts of cowhage (Mucuna pruriens) seeds and anti-snake venom effects. In Nuts & Seeds in Health and Disease Prevention; Victor, R.P., Ronald, R.W., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 401–408. ISBN 9780123756886. [Google Scholar]
- Okafor, A.I.; Nok, A.J.; Inuwa, H. Antiplasmodial activity of aqueous leaf extract of Mucuna pruriens Linn in mice infected with Plasmodium berghei (NK-65 Strain). J. Appl. Pharm. Sci. 2013, 3, S52–S55. [Google Scholar] [CrossRef]
- Shukla, K.K.; Mahdi, A.A.; Ahmad, M.K.; Jaiswar, S.P.; Shankwar, S.N.; Tiwari, S.C. Mucuna pruriens reduces stress and improves the quality of semen in infertile men. Adv. Access Publ. Hindawi. 2010, 7, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Oyeyemi, I.T.; Akinseye, K.M.; Adebayo, S.S.; Oyetunji, M.T.; Oyeyemi, O.T. Ethnobotanical survey of the plants used for the management of malaria in Ondo State, Nigeria. S. Afr. J. Bot. 2019, 124, 391–401. [Google Scholar] [CrossRef]
- Sathiyanarayanan, L.; Arulmozhi, S. Mucuna pruriens Linn.- A comprehensive review. Pharmacogn. Rev. 2007, 1, 157–162. [Google Scholar]
- Lorenzetti, F.; Macisaac, S.; Arnason, J.T.; Awang, D.V.C.; Buckles, D. The phytochemistry, toxicology, and food potential of velvet bean (Mucuna Adans. spp., Fabaceae). In Cover Crops in West Africa: Contributing to Sustainable Agriculture; Daniel, B., Albert, E., Olu, O., Marcel, G., Norma, G., Eds.; IDRC: Ottawa, ON, Canada, 1998; pp. 67–84. ISBN 0-88936-852-X. [Google Scholar]
- Pugalenthi, M.; Vadivel, V.; Siddhuraju, P. Alternative food/feed perspectives of an underutilized legume Mucuna pruriens var. Utilis- A Review. Plant Foods Hum. Nutr. 2005, 60, 201–218. [Google Scholar] [CrossRef]
- Dendup, T.; Prachyawarakorn, V.; Pansanit, A.; Ruchirawat, S.; Kittakoop, P. α-Glucosidase inhibitory activities of isoflavanones, isoflavones, and pterocarpans from Mucuna pruriens. Planta Med. 2014, 80, 604–608. [Google Scholar] [CrossRef] [Green Version]
- e Lacerda, R.R.; Moreira, I.C.; Sabino, J.; Carenina, A.; De Lacerda, S.; Cabral, N.L.; Lucetti, D.L.; Viana, S.D.B.; Francisco, C.; Felipe, B.; et al. Lectin isolated from Brazilian seeds of velvet bean (Mucuna pruriens (L) DC.) presents analgesic, anti- inflammatory and antihemolytic action. J. Med. Plants Res. 2015, 9, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Therapeutic uses of Amaranthus caudatus L. Trop. Biomed. 2019, 36, 1038–1053. [Google Scholar]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Suitability of Amaranthus species for alleviating human dietary deficiencies. S. Afr. J. Bot. 2018, 115, 65–73. [Google Scholar] [CrossRef]
- Olatunji, T.L.; Afolayan, A.J. Comparison of nutritional, antioxidant vitamins and capsaicin contents in Capsicum annuum and C. frutescens. Int. J. Veg. Sci. 2019, 18, 1–18. [Google Scholar] [CrossRef]
- Idih, F.; Ighorodje-Monago, C.; Ezim, O. Antiplasmodial effect of ethanol extract of Morinda lucida and Mucuna pruriens leaves on NK65 chloroquine resistant strain of Plasmodium berghei in mice. J. Clin. Exp. Pharmacol. 2019, 7, 1–4. [Google Scholar] [CrossRef]
- Idris, O.A.; Wintola, O.A.; Afolayan, A.J. Evaluation of the bioactivities of Rumex crispus L. leaves and root extracts using toxicity, antimicrobial, and antiparasitic assays. Evidence-Based Complement. Altern. Med. 2019, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idris, O.A.; Wintola, O.A.; Afolayan, A.J. Phytochemical and antioxidant activities of Rumex crispus L. in treatment of gastrointestinal helminths in Eastern Cape Province, South Africa. Asian Pac. J. Trop. Biomed. 2017, 7, 1071–1078. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Antioxidant and phytochemical activities of Amaranthus caudatus L. harvested from different soils at various growth stages. Sci. Rep. 2019, 9, 12965. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Otunola, G.A.; Afolayan, A.J. Phytochemical screening and in vitro evaluation of antioxidant and antimicrobial activities of Kedrostis africana (L.) Cogn. Asian Pac. J. Trop. Biomed. 2017, 7, 901–908. [Google Scholar] [CrossRef]
- Adegbaju, O.D.; Otunola, G.A.; Afolayan, A.J. Effects of growth stage and seasons on the phytochemical content and antioxidant activities of crude extracts of Celosia argentea L. Heliyon 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Otunola, G.A.; Afolayan, A.J. Polyphenolic content, antioxidant and antimicrobial activities of Vernonia mespilifolia Less. used in folk medicine in the Eastern Cape Province, South Africa. J. Evidence-Based Integr. Med. 2018, 23, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Olatunji, T.L.; Afolayan, A.J. Comparative quantitative study on phytochemical contents and antioxidant activities of Capsicum annuum L. and Capsicum frutescens L. Sci. World J. 2019, 2019, 4705140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Yalavarthi, C.; Thiruvengadarajan, V.S. A review on identification strategy of phyto constituents present in herbal plants. Int. J. Res. Pharm. Sci. 2013, 4, 123–140. [Google Scholar]
- Corns, C.M. Herbal remedies and clinical biochemistry. Ann. Clin. Biochem. 2003, 40, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O.; Otunola, G.A.; Afolayan, A.J. In vitro α-amylase, α-glucosidase, lipase inhibitory and cytotoxic activities of tuber extracts of Kedrostis africana (L.) Cogn. Heliyon 2018, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Nibret, E.; Ashour, M.L.; Rubanza, C.D.; Wink, M. Screening of some Tanzanian medicinal plants for their trypanocidal and cytotoxic activities. Phyther. Res. 2009, 22, 544–549. [Google Scholar] [CrossRef]
- Bizimana, N.; Tietjen, U.; Zessin, K.H.; Diallo, D.; Djibril, C.; Melzig, M.F.; Clausen, P.H. Evaluation of medicinal plants from Mali for their in vitro and in vivo trypanocidal activity. J. Ethnopharmacol. 2006, 103, 350–356. [Google Scholar] [CrossRef]
- Jaberian, H.; Piri, K.; Nazari, J. Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem. 2013, 136, 237–244. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Structural aspects of legume proteins and nutraceutical properties. Food Res. Int. 2015, 76, 19–30. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Bono, A. Phytochemical antioxidants for health and medicine—A move towards nature. Biotechnol. Mol. Biol. Rev. 2007, 1, 97–104. [Google Scholar]
- Dhanasekaran, M.; Tharakan, B.; Manyam, B.V. Antiparkinson Drug—Mucuna pruriens shows antioxidant and metal chelating activity. Phyther. Res. 2008, 22, 6–11. [Google Scholar] [CrossRef] [PubMed]








| S/N | Phytochemicals | Quantity |
|---|---|---|
| 1 | Total phenol | 3730.1 ± 15.52 (mg GAE/g) |
| 2 | Flavonoids | 63.03 ± 1.95 (mg QE/g) |
| 3 | Proanthocyanidins | 18.92 ± 1.09 (mg CE/g) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimoh, M.A.; Idris, O.A.; Jimoh, M.O. Cytotoxicity, Phytochemical, Antiparasitic Screening, and Antioxidant Activities of Mucuna pruriens (Fabaceae). Plants 2020, 9, 1249. https://doi.org/10.3390/plants9091249
Jimoh MA, Idris OA, Jimoh MO. Cytotoxicity, Phytochemical, Antiparasitic Screening, and Antioxidant Activities of Mucuna pruriens (Fabaceae). Plants. 2020; 9(9):1249. https://doi.org/10.3390/plants9091249
Chicago/Turabian StyleJimoh, Mahboob Adekilekun, Oladayo Amed Idris, and Muhali Olaide Jimoh. 2020. "Cytotoxicity, Phytochemical, Antiparasitic Screening, and Antioxidant Activities of Mucuna pruriens (Fabaceae)" Plants 9, no. 9: 1249. https://doi.org/10.3390/plants9091249