The Genome and Transcriptome Analysis of the Vigna mungo Chloroplast
Abstract
:1. Introduction
2. Results and Discussion
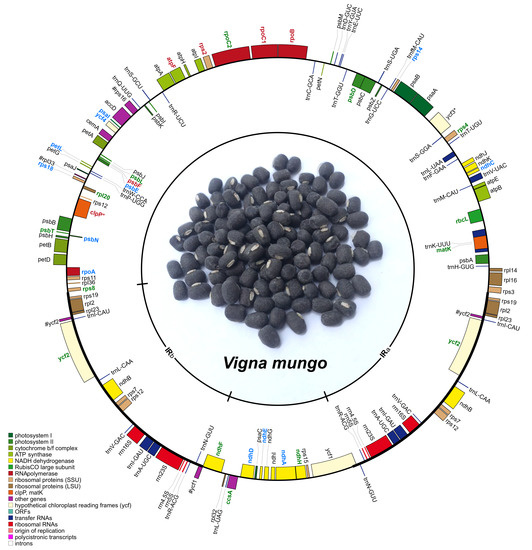
2.1. General Features of Vigna Mungo Chloroplast Genome
2.2. Comparative Chloroplast Genome Analysis
2.3. Positively Selected Genes
2.4. Polycistronic Transcription Units
2.5. RNA Editing
3. Materials and Methods
3.1. DNA and RNA Extraction
3.2. Preparation of DNA and RNA Libraries and Sequencing
3.3. Genome Assembly and Annotation
3.4. Comparative Analysis of V. mungo Chloroplast Genome
3.5. Positive Selection
3.6. RNA Editing Sites
3.7. Polycistronic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Somta, P.; Srinives, P. Genome Research in Mungbean [Vigna radiata (L.) Wilczek] and Blackgram [V. mungo (L.) Hepper]. ScienceAsia 2007, 33, 69. [Google Scholar] [CrossRef]
- Kaewwongwal, A.; Kongjaimun, A.; Somta, P.; Chankaew, S.; Yimram, T.; Srinives, P. Genetic diversity of the black gram [Vigna mungo (L.) Hepper] gene pool as revealed by SSR markers. Breed Sci. 2015, 65, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azeem, F.; Bilal, M.J.; Ijaz, U.; Zubair, M.; Rasul, I.; Asghar, M.J.; Abbas, G.; Atif, R.M.; Hameed, A. Recent Advances in Breeding, Marker Assisted Selection and Genomics of Black Gram (Vigna mungo (L.) Hepper). In Advances in Plant Breeding Strategies: Legumes; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; The Registered Company Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 25–52. [Google Scholar]
- Taberlet, P.; Coissac, E.; Pompanon, F.; Gielly, L.; Miquel, C.; Valentini, A.; Vermat, T.; Corthier, G.; Brochmann, C.; Willerslev, E. Power and limitations of the chloroplast trnL (UAA) intron for plant DNA barcoding. Nucleic Acids Res. 2007, 35, e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Castillo-Ramírez, S.; González, V.; Bustos, P.; Luís Fernández-Vázquez, J.; Santamaría, R.I.; Arellano, J.; Cevallos, M.A.; Dávila, G. Rapid evolutionary change of common bean (Phaseolus vulgaris L) plastome, and the genomic diversification of legume chloroplasts. BMC Genom. 2007, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Saski, C.; Lee, S.-B.; Daniell, H.; Wood, T.C.; Tomkins, J.; Kim, H.-G.; Jansen, R.K. Complete Chloroplast Genome Sequence of Glycine max and Comparative Analyses with other Legume Genomes. Plant Mol. Biol. 2005, 59, 309–322. [Google Scholar] [CrossRef]
- Tangphatsornruang, S.; Sangsrakru, D.; Chanprasert, J.; Uthaipaisanwong, P.; Yoocha, T.; Jomchai, N.; Tragoonrung, S. The Chloroplast Genome Sequence of Mungbean (Vigna radiata) Determined by High-throughput Pyrosequencing: Structural Organization and Phylogenetic Relationships. DNA Res. 2010, 17, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Kaila, T.; Chaduvla, P.K.; Saxena, S.; Bahadur, K.; Gahukar, S.J.; Chaudhury, A.; Sharma, T.R.; Singh, N.K.; Gaikwad, K. Chloroplast Genome Sequence of Pigeonpea (Cajanus cajan (L.) Millspaugh) and Cajanus scarabaeoides (L.) Thouars: Genome Organization and Comparison with Other Legumes. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Palmer, J.D.; Osorio, B.; Aldrich, J.; Thompson, W.F. Chloroplast DNA evolution among legumes: Loss of a large inverted repeat occurred prior to other sequence rearrangements. Curr. Genet. 1987, 11, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-P.; Ko, C.-Y.; Kuo, C.-I.; Liu, M.-S.; Schafleitner, R.; Chen, L.-F.O. Transcriptional Slippage and RNA Editing Increase the Diversity of Transcripts in Chloroplasts: Insight from Deep Sequencing of Vigna radiata Genome and Transcriptome. PLOS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Wang, S.; Xia, E.-H.; Jiang, J.-J.; Zeng, F.-C.; Gao, L.-Z. Full transcription of the chloroplast genome in photosynthetic eukaryotes. Sci. Rep. 2016, 6, 30135. [Google Scholar] [CrossRef]
- Chu, D.; Wei, L. The chloroplast and mitochondrial C-to-U RNA editing in Arabidopsis thaliana shows signals of adaptation. Plant Direct 2019, 3, e00169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.-Z.; Liu, Y.-L.; Zhang, D.; Li, W.; Gao, J.; Liu, Y.; Li, K.; Shi, C.; Zhao, Y.; Zhao, Y.-J.; et al. Evolution of Oryza chloroplast genomes promoted adaptation to diverse ecological habitats. Commun. Biol. 2019, 2, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Stanhope, M.J. Functional bias of positively selected genes in Streptococcus genomes. Infect. Genet. Evolut. 2012, 12, 274–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, D.-F.; Yu, Y.; Deng, Y.-Q.; Li, J.; Liu, H.-Y.; Zhou, S.-D.; He, X.-J. Comparative Analysis of the Chloroplast Genomes of the Chinese Endemic Genus Urophysa and Their Contribution to Chloroplast Phylogeny and Adaptive Evolution. Int. J. Mol. Sci. 2018, 19, 1847. [Google Scholar] [CrossRef] [Green Version]
- Ruhfel, B.R.; Gitzendanner, M.A.; Soltis, P.S.; Soltis, D.E.; Burleigh, J.G. From algae to angiosperms–inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes. BMC Evolut. Biol. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, C.C.; Xi, Z.; Mathews, S. Plastid phylogenomics and green plant phylogeny: Almost full circle but not quite there. BMC Biol. 2014, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.-T.; Yi, T.-S.; Gao, L.-M.; Ma, P.-F.; Zhang, T.; Yang, J.-B.; Gitzendanner, M.A.; Fritsch, P.W.; Cai, J.; Luo, Y.; et al. Origin of angiosperms and the puzzle of the Jurassic gap. Nat. Plants 2019, 5, 461–470. [Google Scholar] [CrossRef]
- Daniell, H.; Lin, C.-S.; Yu, M.; Chang, W.-J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef] [Green Version]
- Shanmugaraj, B.I.; Bulaon, C.J.; Phoolcharoen, W. Plant Molecular Farming: A Viable Platform for Recombinant Biopharmaceutical Production. Plants 2020, 9, 842. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, P.-C.; Chang, W.-J.; Yu, K.; Lin, C.-S. Plastid Transformation: How Does it Work? Can it Be Applied to Crops? What Can it Offer? Int. J. Mol. Sci. 2020, 21, 4854. [Google Scholar] [CrossRef]
- Fouad, W.M.; Altpeter, F. Transplastomic expression of bacterial l-aspartate-α-decarboxylase enhances photosynthesis and biomass production in response to high temperature stress. Transgenic Res. 2009, 18, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Daniell, H. Expression of γ-tocopherol methyltransferase in chloroplasts results in massive proliferation of the inner envelope membrane and decreases susceptibility to salt and metal-induced oxidative stresses by reducing reactive oxygen species. Plant Biotechnol. J. 2014, 12, 1274–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufourmantel, N.; Tissot, G.; Goutorbe, F.; Garçon, F.; Muhr, C.; Jansens, S.; Pelissier, B.; Peltier, G.; Dubald, M. Generation and Analysis of Soybean Plastid Transformants Expressing Bacillus thuringiensis Cry1Ab Protoxin. Plant Mol. Biol. 2005, 58, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Rai, V.; Xiao, Y. Cold chain and virus-free oral polio booster vaccine made in lettuce chloroplasts confers protection against all three poliovirus serotypes. Plant Biotechnol. J. 2019, 17, 1357–1368. [Google Scholar] [CrossRef]
- Arlen, P.A.; Falconer, R.; Cherukumilli, S.; Cole, A.; Cole, A.M.; Oishi, K.K.; Daniell, H. Field production and functional evaluation of chloroplast-derived interferon-α2b. Plant Biotechnol. J. 2007, 5, 511–525. [Google Scholar] [CrossRef] [Green Version]
- Boyhan, D.; Daniell, H. Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide. Plant Biotechnol. J. 2011, 9, 585–598. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, E.N.; Ruhlman, T.A.; Sabir, J.S.M.; Hajrah, N.H.; Alharbi, N.S.; Al-Malki, A.L.; Bailey, C.D.; Jansen, R.K. Plastid genome sequences of legumes reveal parallel inversions and multiple losses of rps16 in papilionoids. J. Syst. Evol. 2015, 53, 458–468. [Google Scholar] [CrossRef]
- Zhu, A.; Guo, W.; Gupta, S.; Fan, W.; Mower, J.P. Evolutionary dynamics of the plastid inverted repeat: The effects of expansion, contraction, and loss on substitution rates. New Phytologist. 2016, 209, 1747–1756. [Google Scholar] [CrossRef] [Green Version]
- Darling, A.C.E.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple Alignment of Conserved Genomic Sequence With Rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [Green Version]
- Jansen, R.K.; Wojciechowski, M.F.; Sanniyasi, E.; Lee, S.-B.; Daniell, H. Complete plastid genome sequence of the chickpea (Cicer arietinum) and the phylogenetic distribution of rps12 and clpP intron losses among legumes (Leguminosae). Mol. Phylogenet. Evolut. 2008, 48, 1204–1217. [Google Scholar] [CrossRef] [Green Version]
- Jeffares, D.C.; Tomiczek, B.; Sojo, V.; dos Reis, M. A Beginners Guide to Estimating the Non-synonymous to Synonymous Rate Ratio of all Protein-Coding Genes in a Genome. In Parasite Genomics Protocols; Peacock, C., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; pp. 65–90. ISBN 978-1-4939-1438-8. [Google Scholar]
- Shi, H.; Yang, M.; Mo, C.; Xie, W.; Liu, C.; Wu, B.; Ma, X. Complete chloroplast genomes of two Siraitia Merrill species: Comparative analysis, positive selection and novel molecular marker development. PLoS ONE 2019, 14, e0226865. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486–487. [Google Scholar] [CrossRef]
- Yang, Z.; Nielsen, R. Codon-Substitution Models for Detecting Molecular Adaptation at Individual Sites Along Specific Lineages. Mol. Biol. Evol. 2002, 19, 908–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClean, P.E.; Lavin, M.; Gepts, P.; Jackson, S.A. Phaseolus vulgaris: A Diploid Model for Soybean. In Genetics and Genomics of Soybean; Stacey, G., Ed.; Plant Genetics and Genomics: Crops and Models; Springer: New York, NY, NY, USA, 2008; pp. 55–76. ISBN 978-0-387-72299-3. [Google Scholar]
- Sen, L.; Fares, M.A.; Liang, B.; Gao, L.; Wang, B.; Wang, T.; Su, Y.-J. Molecular evolution of rbcL in three gymnosperm families: Identifying adaptive and coevolutionary patterns. Biol. Direct 2011, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Simpson, M.G. 8—Diversity and Classification of Flowering Plants: Eudicots. In Plant Systematics, 2nd ed.; Simpson, M.G., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 275–448. ISBN 978-0-12-374380-0. [Google Scholar]
- Sita, K.; Sehgal, A.; HanumanthaRao, B.; Nair, R.M.; Vara Prasad, P.V.; Kumar, S.; Gaur, P.M.; Farooq, M.; Siddique, K.H.M.; Varshney, R.K.; et al. Food Legumes and Rising Temperatures: Effects, Adaptive Functional Mechanisms Specific to Reproductive Growth Stage and Strategies to Improve Heat Tolerance. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Bock, D.G.; Andrew, R.L.; Rieseberg, L.H. On the adaptive value of cytoplasmic genomes in plants. Mol. Ecol. 2014, 23, 4899–4911. [Google Scholar] [CrossRef]
- Kapralov, M.V.; Filatov, D.A. Widespread positive selection in the photosynthetic Rubisco enzyme. BMC Evolut. Biol. 2007, 7, 73. [Google Scholar] [CrossRef] [Green Version]
- Hao, D.C.; Chen, S.L.; Xiao, P.G. Molecular evolution and positive Darwinian selection of the chloroplast maturase matK. J. Plant Res. 2010, 123, 241–247. [Google Scholar] [CrossRef]
- Ivanova, Z.; Sablok, G.; Daskalova, E.; Zahmanova, G.; Apostolova, E.; Yahubyan, G.; Baev, V. Chloroplast Genome Analysis of Resurrection Tertiary Relict Haberlea rhodopensis Highlights Genes Important for Desiccation Stress Response. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Yin, K.; Zhang, Y.; Li, Y.; Du, F.K. Different Natural Selection Pressures on the atpF Gene in Evergreen Sclerophyllous and Deciduous Oak Species: Evidence from Comparative Analysis of the Complete Chloroplast Genome of Quercus aquifolioides with Other Oak Species. Int. J. Mol. Sci. 2018, 19, 1042. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Ario, N.; Nakagawa, A.C.S.; Tomita, Y.; Murayama, N.; Taniguchi, T.; Hamaoka, N.; Iwaya-Inoue, M.; Ishibashi, Y. Effects of light quality on pod elongation in soybean (Glycine max (L.) Merr.) and cowpea (Vigna unguiculata (L.) Walp.). Plant Signal. Behav. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Chotewutmontri, P.; Barkan, A. Dynamics of Chloroplast Translation during Chloroplast Differentiation in Maize. PLoS Genet. 2016, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, R. Structure, function, and inheritance of plastid genomes. In Cell and Molecular Biology of Plastids; Bock, R., Ed.; Topics in Current Genetics; Springer: Berlin, Heidelberg, 2007; pp. 29–63. ISBN 978-3-540-75376-6. [Google Scholar]
- Germain, A.; Hotto, A.M.; Barkan, A.; Stern, D.B. RNA processing and decay in plastids. WIREs RNA 2013, 4, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Stoppel, R.; Meurer, J. Complex RNA metabolism in the chloroplast: An update on the psbB operon. Planta 2013, 237, 441–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meierhoff, K.; Felder, S.; Nakamura, T.; Bechtold, N.; Schuster, G. HCF152, an Arabidopsis RNA Binding Pentatricopeptide Repeat Protein Involved in the Processing of Chloroplast psbB-psbT-psbH-petB-petD RNAs. Plant Cell 2003, 15, 1480–1495. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.-C.; Lee, C.-J.; Chung, Y.-T.; Sung, T.-Y.; Hsieh, M.-H. Differential regulation of Arabidopsis plastid gene expression and RNA editing in non-photosynthetic tissues. Plant Mol. Biol. 2013, 82, 375–392. [Google Scholar] [CrossRef]
- Qulsum, U.; Azad, M.T.A.; Tsukahara, T. Analysis of Tissue-specific RNA Editing Events of Genes Involved in RNA Editing in Arabidopsis thaliana. J. Plant Biol. 2019, 62, 351–358. [Google Scholar] [CrossRef]
- Shikanai, T.; Shimizu, K.; Ueda, K.; Nishimura, Y.; Kuroiwa, T.; Hashimoto, T. The Chloroplast clpP Gene, Encoding a Proteolytic Subunit of ATP-Dependent Protease, is Indispensable for Chloroplast Development in Tobacco. Plant Cell Physiol. 2001, 42, 264–273. [Google Scholar] [CrossRef]
- Chateigner-Boutin, A.-L.; Ramos-Vega, M.; Guevara-García, A.; Andrés, C.; Gutiérrez-Nava, M.D.L.L.; Cantero, A.; Delannoy, E.; Jiménez, L.F.; Lurin, C.; Small, I.; et al. CLB19, A pentatricopeptide repeat protein required for editing of rpoA and clpP chloroplast transcripts. Plant J. 2008, 56, 590–602. [Google Scholar] [CrossRef]
- Hein, A.; Knoop, V. Expected and unexpected evolution of plant RNA editing factors CLB19, CRR28 and RARE1: Retention of CLB19 despite a phylogenetically deep loss of its two known editing targets in Poaceae. BMC Evolut. Biol. 2018, 18, 85. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Wu, Y.; Maliga, P.; Messing, J. RNA Editing in Chloroplasts of Spirodela polyrhiza, an Aquatic Monocotelydonous Species. PLOS ONE 2015, 10, e0140285. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, M.; Sugita, M. RNA Editing and Its Molecular Mechanism in Plant Organelles. Genes 2017, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Yang, C.; Zhao, X.; Chen, S.; Qu, G.-Z. Complete chloroplast genome sequence of Betula platyphylla: Gene organization, RNA editing, and comparative and phylogenetic analyses. BMC Genom. 2018, 19, 950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillich, M.; Lehwark, P.; Morton, B.R.; Maier, U.G. The Evolution of Chloroplast RNA Editing. Mol. Biol. Evol. 2006, 23, 1912–1921. [Google Scholar] [CrossRef] [Green Version]
- Pootakham, W.; Sonthirod, C.; Naktang, C.; Ruang-Areerate, P.; Yoocha, T.; Sangsrakru, D.; Theerawattanasuk, K.; Rattanawong, R.; Lekawipat, N.; Tangphatsornruang, S. De novo hybrid assembly of the rubber tree genome reveals evidence of paleotetraploidy in Hevea species. Sci. Rep. 2017, 7, 41457. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-J.; Yu, W.-B.; Yang, J.-B.; Song, Y.; dePamphilis, C.W.; Yi, T.-S.; Li, D.-Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. bioRxiv 2019, 256479. [Google Scholar] [CrossRef] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput Biol 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Chen, H.; Jiang, M.; Wang, L.; Wu, X.; Huang, L.; Liu, C. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res. 2019, 47, W65–W73. [Google Scholar] [CrossRef]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq–versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. IRscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics 2018, 34, 3030–3031. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Peterson, D.; Tamura, K. MEGA-CC: Computing core of molecular evolutionary genetics analysis program for automated and iterative data analysis. Bioinformatics 2012, 28, 2685–2686. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z. PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Chen, C.; Arab, D.A.; Du, Z.; He, Y.; Ho, S.Y.W. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 2019, 9, 3891–3898. [Google Scholar] [CrossRef] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Picardi, E.; Pesole, G. REDItools: High-throughput RNA editing detection made easy. Bioinformatics 2013, 29, 1813–1814. [Google Scholar] [CrossRef] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]




| Category of Genes | Group of Genes | Name of Genes |
|---|---|---|
| Genes for photosynthesis | Subunits of photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ, ycf3 ** |
| Subunits of photosystem I | psaA, psaB, psaC, psaI, psaJ | |
| Subunits of NADH-dehydrogenase | ndhA *, ndhB *, ndhC, ndh, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| Subunits of cytochrome b/f complex | petA, petB *, petD *, petG, petL, petN | |
| Subunits of ATP synthase | atpA, atpB, atpE, atpF *, atpH, atpI | |
| Subunit of rubisco | rbcL | |
| Self-replication | Large subunit of ribosome | rpl14, rpl16 *, rpl2, rpl20, rpl23, rpl32, rpl36, rpl33ψ |
| DNA dependent RNA polymerase | rpoA, rpoB, rpoC1 *, rpoC2 | |
| Small subunit of ribosome | rps11, rps12 *, rps14, rps15, rps16 *,ψ, rps18, rps19, rps2, rps3, rps4, rps7, rps8 | |
| Other genes | Subunit of Acetyl-CoA-carboxylase | accD |
| c-type cytochrom synthesis gene | ccsA | |
| Envelop membrane protein | cemA | |
| Protease | clpP ** | |
| Maturase | matK | |
| Ribosomal RNAs | rrn23S, rrn4.5S, rrn16S, rrn5S | |
| Transfer RNAs | Ala | TrnA-UGC * |
| Arg | trnR-ACG, trnR-UCU | |
| Asn | trnN-GUU | |
| Asp | trnD-GUC | |
| Cys | trnC-GCA | |
| Gln | trnQ-UUG | |
| Glu | trnE-UUC | |
| Gly | trnG-UCC | |
| His | trnH-GUG | |
| Ile | trnI-CAU, trnI-GAU * | |
| Leu | trnL-CAA, trnL-UAA *, trnL-UAG | |
| Lys | trnK-UUU * | |
| Met | trnM-CAU, trnfM-CAU | |
| Phe | trnF-GAA | |
| Pro | trnP-UGG | |
| Ser | trnS-GCU, trnS-GGA, trnS-UGA | |
| Thr | trnT-GGU, trnT-UGU | |
| Trp | trnW-CCA | |
| Tyr | TrnY-GUA | |
| Val | trnV-GAC, trnV-UAC * | |
| Unkown | Conserved open reading frames | ycf1, ycf1 ψ, ycf2, ycf4 |
| Gene | Function | Ka/Ks of Gene | LRTs (2ΔLnL) | LRT p-Value | Selective Site | Pr(Ka/Ks > 1) | Ka/Ks of Site |
|---|---|---|---|---|---|---|---|
| atpF | Photosynthesis | 1.94 | 0.39 | 0.02 | 39R | 0.967 * | 1.884 |
| 62N | 0.996 ** | 1.931 | |||||
| 79T | 0.971 * | 1.89 | |||||
| 83L | 0.952 * | 1.86 | |||||
| 167M | 0.984 * | 1.912 | |||||
| ccsA | Other genes | 2.40 | 0.64 | 0.00 | 94Q | 0.982 * | 2.048 |
| clpP | Other genes | 2.87 | 0.83 | 0.04 | 12I | 0.983 * | 2.836 |
| matK | Other genes | 2.82 | 0.07 | 0.00 | 80V | 0.956 * | 2.725 |
| 493Y | 0.988 * | 3.08 | |||||
| 494L | 0.989 * | 3.083 | |||||
| ndhF | Photosynthesis | 1.55 | 0.55 | 0.00 | 64K | 0.989 * | 1.54 |
| 289K | 0.995 ** | 1.546 | |||||
| 507I | 0.993 ** | 1.544 | |||||
| 616L | 0.965 * | 1.512 | |||||
| 638L | 0.989 * | 1.54 | |||||
| 740N | 0.951 * | 1.497 | |||||
| 741K | 0.951 * | 1.496 | |||||
| ndhH | Photosynthesis | 1.03 | 0.15 | 0.00 | 3I | 0.955 * | 1.502 |
| 176S | 0.994 ** | 1.026 | |||||
| 269I | 0.989 * | 1.021 | |||||
| 294C | 0.986 * | 1.019 | |||||
| psbD | Photosynthesis | 2.17 | 0.25 | 0.01 | 122G | 1.000 ** | 2.168 |
| psbE | Photosynthesis | 3.94 | 0.17 | 0.05 | 59N | 0.999 ** | 3.937 |
| psbL | Photosynthesis | 5.27 | 0.14 | 0.00 | 1M | 1.000 ** | 5.267 |
| psbT | Photosynthesis | 1.71 | 0.01 | 0.00 | 28K | 1.000 ** | 1.711 |
| 33K | 1.000 ** | 1.711 | |||||
| 34V | 0.952 * | 3.873 | |||||
| rbcL | Photosynthesis | 1.64 | 0.16 | 0.00 | 28D | 0.985 * | 1.613 |
| 86H | 1.000 ** | 3.108 | |||||
| 95S | 0.993 ** | 1.624 | |||||
| 97F | 0.999 ** | 1.633 | |||||
| 142T | 0.999 ** | 3.106 | |||||
| 228S | 0.958 * | 3.001 | |||||
| 251M | 0.999 ** | 1.634 | |||||
| 375I | 0.990 * | 1.619 | |||||
| 449S | 0.997 ** | 3.102 | |||||
| 470E | 0.999 ** | 1.634 | |||||
| 475I | 0.998 ** | 3.105 | |||||
| rpl20 | subunit of ribosome | 2.92 | 0.06 | 0.05 | 84K | 0.979 * | 3.167 |
| rpoB | RNA polymerase | 1.49 | 0.62 | 0.00 | 446I | 0.965 * | 1.473 |
| rpoC1 | RNA polymerase | 1.30 | 1.39 | 0.00 | 562W | 0.991 ** | 1.294 |
| 568P | 0.964 * | 1.472 | |||||
| 569K | 0.973 * | 1.275 | |||||
| rpoC2 | RNA polymerase | 2.75 | 0.65 | 0.00 | 734S | 0.990 * | 2.728 |
| 735K | 0.980 * | 2.706 | |||||
| rps2 | subunit of ribosome | 1.39 | 0.82 | 0.00 | 67G | 0.998 ** | 1.384 |
| 129F | 0.954 * | 1.332 | |||||
| 130Q | 0.960 * | 1.339 | |||||
| 131S | 0.959 * | 1.337 | |||||
| 235S | 0.991 ** | 1.376 | |||||
| rps4 | subunit of ribosome | 3.81 | 0.68 | 0.00 | 25K | 0.983 * | 4.044 |
| 99A | 0.956 * | 3.661 | |||||
| rps8 | subunit of ribosome | 1.54 | 1.11 | 0.02 | 55N | 0.969 * | 1.502 |
| 93Q | 0.996 ** | 1.537 | |||||
| ycf2 | Other genes | 3.31 | 0.05 | 0.00 | 3G | 0.986 * | 2.481 |
| 117S | 0.992 ** | 2.491 | |||||
| 120S | 0.963 * | 2.444 | |||||
| 429R | 0.958 * | 2.435 | |||||
| 484Q | 0.962 * | 2.441 | |||||
| 627V | 0.997 ** | 2.498 | |||||
| 692K | 0.984 * | 2.478 | |||||
| 693T | 0.966 * | 2.448 | |||||
| 716T | 0.960 * | 2.439 | |||||
| 1040L | 0.976 * | 2.465 | |||||
| 1528S | 0.982 * | 2.474 |
| Tissue | Gene | Position on Gene | Base Change | Amino Acid Change | Editing Efficiency * | Codon Position | Coverage |
|---|---|---|---|---|---|---|---|
| Leaf | ndhC | 323 | C -> T | S -> L | 0.89 | 2 | 27 |
| Leaf | rps14 | 80 | C -> T | S -> L | 0.9 | 2 | 20 |
| Leaf | rpoB | 551 | C -> T | S -> L | 0.53 | 2 | 19 |
| Leaf | rpoB | 566 | C -> T | S -> L | 0.61 | 2 | 18 |
| Leaf | rpoC1 | 41 | C -> T | S -> L | 0.75 | 2 | 16 |
| Leaf | rps2 | 134 | C -> T | T -> I | 0.95 | 2 | 64 |
| Shoot | rps2 | 134 | C -> T | T -> I | 0.95 | 2 | 21 |
| Root | rps2 | 134 | C -> T | T -> I | 0.93 | 2 | 15 |
| Leaf | rps2 | 248 | C -> T | S -> L | 0.81 | 2 | 16 |
| Leaf | atpF | 92 | C -> T | P -> L | 0.92 | 2 | 38 |
| Leaf | psaI | 79 | C -> T | H -> Y | 0.78 | 1 | 54 |
| Leaf | psbF | 44 | C -> T | S -> F | 0.7 | 2 | 37 |
| Leaf | psbE | 124 | C -> T | P -> L | 0.92 | 1 | 34 |
| Shoot | psbE | 124 | C -> T | P -> L | 0.88 | 1 | 21 |
| Leaf | petL | 5 | C -> T | P -> L | 0.43 | 2 | 20 |
| Leaf | rps18 | 221 | C -> T | S -> L | 0.67 | 2 | 26 |
| Leaf | clpP | 2041 | C -> T | H -> Y | 0.82 | 1 | 16 |
| Shoot | clpP | 2041 | C -> T | H -> Y | 0.71 | 1 | 23 |
| Flower | clpP | 2041 | C -> T | H -> Y | 0.74 | 1 | 36 |
| Pod | clpP | 2041 | C -> T | H -> Y | 0.39 | 1 | 100 |
| Root | clpP | 2041 | C -> T | H -> Y | 0.66 | 1 | 69 |
| Leaf | psbN | 104 | C -> T | S -> F | 0.31 | 2 | 78 |
| Flower | psbN | 104 | C -> T | S -> F | 0.47 | 2 | 54 |
| Leaf | rpoA | 803 | C -> T | S -> L | 0.32 | 2 | 32 |
| Leaf | ndhD | 620 | C -> T | S -> L | 0.28 | 2 | 61 |
| Leaf | ndhD | 824 | C -> T | S -> L | 0.35 | 2 | 15 |
| Leaf | ndhD | 1115 | C -> T | T -> I | 0.67 | 2 | 31 |
| Leaf | ndhE | 74 | C -> T | P -> L | 0.95 | 2 | 18 |
| Leaf | ndhA | 20 | C -> T | S -> F | 0.85 | 2 | 40 |
| Leaf | ndhA | 341 | C -> T | S -> L | 0.82 | 2 | 42 |
| Shoot | ndhA | 341 | C -> T | S -> L | 0.48 | 2 | 22 |
| Leaf | ycf4 | 20 | G -> A | R -> K | 0.14 | 2 | 55 |
| Leaf | ycf4 | 309 | G -> A | K -> K | 0.24 | 3 | 40 |
| Leaf | ycf4 | 316 | G -> A | E -> K | 0.24 | 1 | 21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawae, W.; Yundaeng, C.; Naktang, C.; Kongkachana, W.; Yoocha, T.; Sonthirod, C.; Narong, N.; Somta, P.; Laosatit, K.; Tangphatsornruang, S.; et al. The Genome and Transcriptome Analysis of the Vigna mungo Chloroplast. Plants 2020, 9, 1247. https://doi.org/10.3390/plants9091247
Nawae W, Yundaeng C, Naktang C, Kongkachana W, Yoocha T, Sonthirod C, Narong N, Somta P, Laosatit K, Tangphatsornruang S, et al. The Genome and Transcriptome Analysis of the Vigna mungo Chloroplast. Plants. 2020; 9(9):1247. https://doi.org/10.3390/plants9091247
Chicago/Turabian StyleNawae, Wanapinun, Chutintorn Yundaeng, Chaiwat Naktang, Wasitthee Kongkachana, Thippawan Yoocha, Chutima Sonthirod, Nattapol Narong, Prakit Somta, Kularb Laosatit, Sithichoke Tangphatsornruang, and et al. 2020. "The Genome and Transcriptome Analysis of the Vigna mungo Chloroplast" Plants 9, no. 9: 1247. https://doi.org/10.3390/plants9091247